This is a preprint.
SARS-CoV-2 antibody immunity across three continents: the West Africa, West Indies, West London Consortium
- PMID: 40585115
- PMCID: PMC12204446
- DOI: 10.1101/2025.06.13.25329588
SARS-CoV-2 antibody immunity across three continents: the West Africa, West Indies, West London Consortium
Abstract
Background: The experience of the COVID-19 pandemic has differed across continents. We hypothesized that regional differences in SARS-CoV-2 immunity might explain this observation. We therefore established the WWW Consortium in Ghana, W Africa; Jamaica, W Indies; and W London. Here, we describe the extent to which antibody immunity differs between these geographic locations.
Methods: The WWW Consortium harmonises across the HERITAGE (Accra, Ghana), WINDFall (Kingston, Jamaica) and Legacy (London, UK) studies, establishing sharing frameworks for samples, metadata, and data; related permissions and oversight; and associated physical and cloud infrastructure. With centralised testing, we performed serological assessments across all three locations at two snapshots in 2024 (April 1st - August 18th; August 19th - December 31st) using high-throughput live virus neutralization and anti-nucleocapsid IgG, including n=763 individuals.
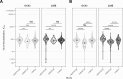
Findings: We found that across all sites most participants had detectable neutralising antibody titres against JN.1 and XEC - the predominant variants in 2024. There were site-related differences in immunity: vaccine-included SARS-CoV-2 strains were better neutralised by participants from the Legacy study - Ancestral, BA.5, XBB.1.5 initially, and JN.1 after a homologous booster in autumn 2024. For HERITAGE, neutralisation of both alpha- (HCoV-229E) and beta-coronaviruses (HCoV-OC43) was higher than WINDFall suggesting a cross-coronavirus serological response in West Africa. Finally, antigenic cartography identified two distinct antibody landscapes, with JN.1 and XEC antigenically distant in Legacy, but not in HERITAGE and WINDFall.
Interpretation: There is international heterogeneity in SARS-CoV-2 antibody immunity. Global recommendations for vaccine strain selection should incorporate data from diverse populations to ensure accurate, equitable recommendations.
Funding: The Wellcome Trust.
Conflict of interest statement
Declarations of interests YB and JMN & DH within the HERITAGE study team own shares of Yemaachi Biotech. ECW reports consulting for AstraZeneca and CSL-Seqirus unrelated to this work. DLVB reports discussions between the Francis Crick institute and GSK for SARS-CoV-2 antiviral testing, and grants to the Crick from AstraZeneca unrelated to this work. Declarations from other consortium authors deemed not relevant to this work.
Figures




References
-
- Choe YJ, Blatt DB, Lee HJ, Choi EH. Associations between geographic region and immune response variations to pneumococcal conjugate vaccines in clinical trials: A systematic review and meta-analysis. Int J Infect Dis. 2020. Mar;92:261–8. - PubMed
-
- Höhler T, Reuss E, Freitag CM, Schneider PM. A functional polymorphism in the IL-10 promoter influences the response after vaccination with HBsAg and hepatitis A. Hepatology. 2005. Jul;42(1):72–6. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
