This is a preprint.
FUS Mislocalization Rewires a Cortical Gene Network to Drive Cognitive and Behavioral Impairment in ALS
- PMID: 40585174
- PMCID: PMC12204245
- DOI: 10.1101/2025.06.16.25329673
FUS Mislocalization Rewires a Cortical Gene Network to Drive Cognitive and Behavioral Impairment in ALS
Abstract
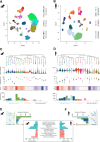
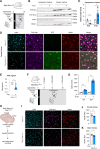
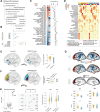
Cognitive and behavioral impairment affects up to half of individuals with amyotrophic lateral sclerosis (ALS), but their molecular origin remains unresolved. Here, we identify mislocalization of the RNA-binding protein FUS in cortical neurons as a defining feature in ALS patients with cognitive impairment (ALS-ci). Selective mislocalization of FUS in adult cortical projection neurons in mice is sufficient to trigger ALS-ci- and ALS with behavioral impairment (ALS-bi)-like phenotypes, including deficits in sociability, and neurodegeneration. Single-nucleus transcriptomics reveal a conserved FUS-dependent gene network downregulated in these mice and ALS-ci patients. This regulon is enriched for ALS genetic risk factors and newly implicates FBXO16 in ALS-bi. Carriers of protein-truncating FBXO16 variants display behavioral abnormalities, frontotemporal atrophy, and increased levels of dementia-linked biomarkers. These findings define a neuron-intrinsic mechanism for cognitive and behavioral dysfunction in ALS and nominate FUS mislocalization and its downstream gene network as therapeutic targets.
Keywords: Amyotrophic lateral sclerosis; behavioral impairment; cognitive impairment; fronto-temporal dementia; genetics; mouse models; single cell biology.
Conflict of interest statement
Declaration of interests BJT has a patent pending (U.S. Patent Application No. 63/717,807) on the diagnostic testing for ALS based on a proteomic panel. BJT holds patents on the clinical testing and therapeutic intervention for the hexanucleotide repeat expansion ofC9orf72. BJT receives research support from Cerevel Therapeutics.
Figures




References
-
- Abrahams S. (2023). Neuropsychological impairment in amyotrophic lateral sclerosis-frontotemporal spectrum disorder. Nat Rev Neurol 19, 655–667. - PubMed
-
- Aguzzoli C.S., Battista P., Hadad R., Ferreira Felloni Borges Y., Schilling L.P., and Miller B.L. (2022). Very early-onset behavioral variant frontotemporal dementia in a patient with a variant of uncertain significance of a FUS gene mutation. Neurocase 28, 403–409. - PubMed
-
- Baxi E.G., Thompson T., Li J., Kaye J.A., Lim R.G., Wu J., Ramamoorthy D., Lima L., Vaibhav V., Matlock A., et al. (2022). Answer ALS, a large-scale resource for sporadic and familial ALS combining clinical and multi-omics data from induced pluripotent cell lines. Nat Neurosci 25, 226–237. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
