Sirtuin 4 accelerates heart failure development by enhancing reactive oxygen species-mediated profibrotic transcriptional signaling
- PMID: 40585275
- PMCID: PMC12206323
- DOI: 10.1016/j.jmccpl.2025.100299
Sirtuin 4 accelerates heart failure development by enhancing reactive oxygen species-mediated profibrotic transcriptional signaling
Abstract
Aims: Sirtuin 4 (SIRT4) is a mitochondrially-localized stress-responsive NAD+-dependent deacetylase predominantly regulating energy metabolism and reactive oxygen species (ROS) homeostasis. Overexpression of SIRT4 aggravates angiotensin-induced cardiac hypertrophy, however underlying mechanisms remain incompletely elucidated. To current study was designed to explore mechanisms underlying adverse effects of increased SIRT4 levels in the heart following pressure overload.
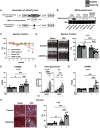
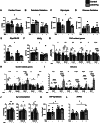
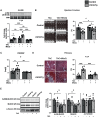
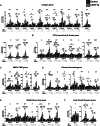
Methods and results: Mice with cardiomyocyte-specific overexpression of Sirt4 (cSirt4-Tg) or non-transgenic controls underwent transverse aortic constriction (TAC) or sham procedure. Cardiac structure, function and energy metabolism were assessed by echocardiography and working heart perfusions. Transcriptome analysis was performed using RNA sequencing. Nine weeks following TAC and thereafter, cSirt4-Tg mice displayed exacerbated cardiac dilation, dysfunction, and fibrosis compared to non-transgenic controls. This aggravation was accompanied by impaired rates of glycolysis and a blunted increase of mitochondrial respiratory capacity. More importantly, expression of numerous genes encoding collagens and profibrotic regulators was elevated. This profibrotic signaling was reversed by mitochondria-targeted antioxidant treatment using MitoQ, along with attenuation of cardiac dysfunction and reversal of structural remodeling. SIRT4 may drive oxidative stress and fibrotic signaling via increased NOX4 expression (>7-fold), and/or direct modulation of potential SIRT4 targets newly identified by Human Protein Microarray, including calcitonin gene-related peptide receptor component protein, cyclophilin A, and interleukin-2 receptor β.
Conclusions: SIRT4 overexpression accelerates heart failure development in response to pressure overload, predominantly by ROS-mediated enhancement of profibrotic transcriptional signaling.
Keywords: Fibrosis; Heart failure; Oxidative stress; Reactive oxygen species; SIRT4; Sirtuin.
© 2025 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Heiko Bugger reports financial support was provided by Austrian Science Fund, German Research Foundation. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- J.N. Cohn, R. Ferrari, N. Sharpe, Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling, J Am Coll Cardiol 35(3) (2000) 569–82. - PubMed
-
- Bugger H., Witt C.N., Bode C. Mitochondrial sirtuins in the heart. Heart Fail Rev. 2016;21(5):519–528. - PubMed
-
- Haigis M.C., Mostoslavsky R., Haigis K.M., Fahie K., Christodoulou D.C., Murphy A.J., et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126(5):941–954. - PubMed
-
- Luo Y.X., Tang X., An X.Z., Xie X.M., Chen X.F., Zhao X., et al. SIRT4 accelerates Ang II-induced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity. Eur Heart J. 2017;38(18):1389–1398. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

