Angiotensin AT1R expressing cells within the ventral hypothalamus modulate integrative control of cardiometabolic functions
- PMID: 40585361
- PMCID: PMC12205624
- DOI: 10.1016/j.isci.2025.112797
Angiotensin AT1R expressing cells within the ventral hypothalamus modulate integrative control of cardiometabolic functions
Abstract
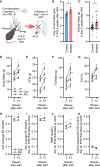
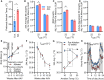
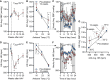
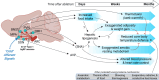
Subtypes of agouti-related peptide (AgRP) neurons can be distinguished based upon expression of angiotensin II receptors. The type 1 subtype, which expresses the angiotensin type 1 receptor (Agtr1a), was examined for its role in the integrative control of cardiometabolic functions. Mice expressing Cre-recombinase via the Agtr1a locus received bilateral microinjection of an AAV vector encoding Cre-dependent expression of caspase-3 into the arcuate nucleus of mice housed at 22°C or 30°C. Ablation of Agtr1a + cells caused increased food intake, adiposity, resting metabolic rate and shifts in nutrient partitioning, elevated temperature preference, loss of core temperature defense, and changes in blood pressure and heart rate. Many phenotypes were ameliorated by housing animals at thermoneutrality. Thus, Agtr1a + cells in the ventral hypothalamus (including the type 1 AgRP neuron) influence cardiometabolic control through modulation of thermoregulatory responses to cold. Some functions of these cells appear to oppose canonical roles of other AgRP neuron subtypes.
Keywords: Molecular biology; Neuroscience; Omics.
© 2025 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Fothergill E., Guo J., Howard L., Kerns J.C., Knuth N.D., Brychta R., Chen K.Y., Skarulis M.C., Walter M., Walter P.J., Hall K.D. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring, Md.) 2016;24:1612–1619. doi: 10.1002/oby.21538. - DOI - PMC - PubMed
-
- Sapouckey S.A., Morselli L.L., Deng G., Patil C.N., Balapattabi K., Oliveira V., Claflin K.E., Gomez J., Pearson N.A., Potthoff M.J., et al. Exploration of cardiometabolic and developmental significance of angiotensinogen expression by cells expressing the leptin receptor or agouti-related peptide. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020;318:R855–R869. doi: 10.1152/ajpregu.00297.2019. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

