Emerging technologies towards extracellular vesicles large-scale production
- PMID: 40585384
- PMCID: PMC12206051
- DOI: 10.1016/j.bioactmat.2025.06.005
Emerging technologies towards extracellular vesicles large-scale production
Abstract
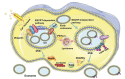
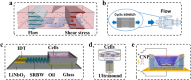
Extracellular vesicles (EVs), which carry bioactive components such as proteins and nucleic acids, reflect the physiological state of their parent cells and play a key role in mediating complex intercellular signaling. Leveraging these unique characteristics, researchers have explored their potential applications in cell therapy, non-invasive biopsies, and tissue regeneration. Therefore, standardized and scalable methods for EVs production and purification are crucial for clinical application and therapeutic settings. However, the limited yields of traditional production and isolation methods have hampered full potential of EVs. In this review, we will introduce strategies aimed at enhancing EV production include optimizing cell yield, expanding cell culture scale, and exploring alternative EVs production sources such as non-mammalian organisms and artificially produced vesicles. Various approaches as well as the bioreactors for controlling cell culture to enhance EVs production, will be introduced in detail. These approaches include regulation of culture parameters, culture medium components, and external stimuli. Additionally, the comparison between traditional ultracentrifugation methods and advance microfluidic isolating methods will be analyzed and discussed. Finally, we will introduce the potential challenges of transitioning EVs from basic research to clinical application and further discuss the future prospects. As the technology advances and different methods are integrated, there is significant potential to enable large-scale EVs production and improve their clinical translation.
Keywords: Bioreactor; Clinical translation; Extracellular vesicles; Isolation; Large-scale.
© 2025 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Yuanjin Zhao reports financial support was provided by National Natural Science Foundation of China. Yuanjin Zhao reports financial support was provided by National Key Research and Development Program of China. Yuanjin Zhao reports financial support was provided by Guangdong Basic and Applied Basic Research Foundation. Yuanjin Zhao reports financial support was provided by Clinical Trials from Nanjing Drum Tower Hospital. Yuanjin Zhao reports financial support was provided by Shenzhen Fundamental Research Program. Yuanjin Zhao is an editorial board member for Bioactive Materials and was not involved in the editorial review or the decision to publish this article. Other authors declare that there are no competing interests.
Figures









Similar articles
-
Physicochemical Modulation Strategies for Mass Production of Extracellular Vesicle.Tissue Eng Regen Med. 2025 Jul;22(5):569-591. doi: 10.1007/s13770-025-00726-9. Epub 2025 Jun 5. Tissue Eng Regen Med. 2025. PMID: 40471522 Free PMC article. Review.
-
Brain-derived extracellular vesicles: A promising avenue for Parkinson's disease pathogenesis, diagnosis, and treatment.Neural Regen Res. 2026 Apr 1;21(4):1447-1467. doi: 10.4103/NRR.NRR-D-24-01262. Epub 2025 Apr 29. Neural Regen Res. 2026. PMID: 40313118
-
Extracellular Vesicle-Integrated Biomaterials in Bone Tissue Engineering Applications: Current Progress and Future Perspectives.Int J Nanomedicine. 2025 Jun 17;20:7653-7683. doi: 10.2147/IJN.S522198. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40546799 Free PMC article. Review.
-
Extracellular Vesicles: Recent Advances and Perspectives.Front Biosci (Landmark Ed). 2025 May 30;30(6):36405. doi: 10.31083/FBL36405. Front Biosci (Landmark Ed). 2025. PMID: 40613286 Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
Cited by
-
Extracellular Vesicles Containing MDP Derived from Lactobacillus rhamnosus GG Inhibit HSV-2 Infection by Activating the NOD2-IFN-I Signalling Pathway.J Extracell Vesicles. 2025 Aug;14(8):e70152. doi: 10.1002/jev2.70152. J Extracell Vesicles. 2025. PMID: 40825575 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

