Fecal Microbiota Transplantation (FMT) in Clostridium difficile Infection: A Paradigm Shift in Gastrointestinal Microbiome Modulation
- PMID: 40585700
- PMCID: PMC12206541
- DOI: 10.7759/cureus.85054
Fecal Microbiota Transplantation (FMT) in Clostridium difficile Infection: A Paradigm Shift in Gastrointestinal Microbiome Modulation
Abstract
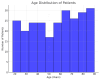
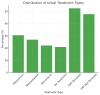
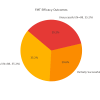
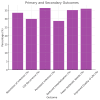
Clostridium difficile (C. difficile) infection (CDI) poses a tremendous clinical challenge, especially in patients with recurrent disease and antibiotic resistance. Fecal microbiota transplantation (FMT) has become a new therapeutic strategy for restoring gut microbiota and decreasing CDI recurrence. The study aims to assess the clinical effectiveness of FMT in adult subjects with recurrent or refractory CDI, determine its effect on gut microbiome diversity, and track safety outcomes and rates of recurrence post-treatment. FMT was compared against standard antibiotic treatments to establish its efficacy in decreasing infection persistence and improving patients' quality of life. This study examines the efficacy, safety, and modulation of microbiota by FMT in an ensemble of 250 patients diagnosed with CDI, with equal gender distribution and a mean age of 55.61. Among the study participants, 131 (52.4%) underwent FMT by various routes of administration, including 66 (25.2%) through colonoscopy, 73 (29.2%) via a nasogastric tube, 60 (24.0%) via enema, and 54 (21.6%) through oral capsule administration. The success rate for FMT was reported as 88 (35.2%), partial success at 74 (29.6%), and treatment failure at 88 (35.2%). CDI recurrence was reported in 130 (52.0%) of patients after FMT. The gut microbiome enhanced diversity, measured in terms of the Shannon Diversity Index, increased significantly from 3.96 before FMT to 5.88 after FMT, thus indicating a favorable impact on gut microbial composition. Furthermore, 132 (52.8%) converted from C. difficile polymerase chain reaction (PCR) toxin positive to negative, corroborating successful pathogen clearance. On secondary outcomes, the quality of life in patients improved in 90 (36%), antibiotic dependence was reduced in 88 (35.2%), and hospitalization was lessened in 72 (28.8%). Inflammatory markers, such as white blood cell (WBC) counts and C-reactive protein (CRP), went downward but did not reach statistical significance. Logistic regression analysis identified age, severity of CDI, and prior exposure to antibiotics as the main predictors for the efficacy of FMT (p < 0.05). It is concluded that FMT is a promising alternative treatment for recurrent CDI through modulation of gut microbiota and decreasing the severity of infection. Future work is, however, required to establish treatment protocols with optimized results for long-term effectiveness and minimized recurrence risks.
Keywords: clostridium difficile infection; fecal microbiota transplantation; gut microbiome; microbiome diversity; recurrent cdi.
Copyright © 2025, Hamza Saeed et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Abbas Institute of Medical Sciences issued approval 11820AIMS/2023. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
Similar articles
-
Fecal microbiota transplantation for the treatment of recurrent Clostridioides difficile (Clostridium difficile).Cochrane Database Syst Rev. 2023 Apr 25;4(4):CD013871. doi: 10.1002/14651858.CD013871.pub2. Cochrane Database Syst Rev. 2023. PMID: 37096495 Free PMC article.
-
Intestinal Microbiota and Fecal Transplantation in Patients with Inflammatory Bowel Disease and Clostridioides difficile: An Updated Literature Review.J Clin Med. 2025 Jul 25;14(15):5260. doi: 10.3390/jcm14155260. J Clin Med. 2025. PMID: 40806882 Review.
-
Host origin of microbiota drives functional recovery and Clostridioides difficile clearance in mice.mBio. 2025 Jul 9;16(7):e0110825. doi: 10.1128/mbio.01108-25. Epub 2025 Jun 2. mBio. 2025. PMID: 40454811 Free PMC article.
-
Faecal microbiota transplantation for recurrent Clostridiodes difficile infection & its global regulatory landscape.Indian J Med Res. 2025 Feb;161(2):113-119. doi: 10.25259/IJMR_818_2024. Indian J Med Res. 2025. PMID: 40257135 Free PMC article. Review.
-
Fecal Microbiota Transplantation for Clostridium difficile Infection: A Systematic Review.Ann Intern Med. 2015 May 5;162(9):630-8. doi: 10.7326/M14-2693. Ann Intern Med. 2015. PMID: 25938992
References
-
- A randomized controlled trial of efficacy and safety of fecal microbiota transplant for preventing recurrent Clostridioides difficile infection. Drekonja DM, Shaukat A, Huang Y, et al. Clin Infect Dis. 2025;80:52–60. - PubMed
-
- 'Does this fecal microbiota transplant work?' Quality assurance of capsule based fecal microbiota transplant production. Jochumsen EA, Kragsnaes MS, Nilsson AC, et al. Scand J Gastroenterol. 2024;59:1234–1239. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
