Immuno-oncological interactions between meningeal lymphatics and glioblastoma: from mechanisms to therapies
- PMID: 40585979
- PMCID: PMC12203814
- DOI: 10.7150/thno.111972
Immuno-oncological interactions between meningeal lymphatics and glioblastoma: from mechanisms to therapies
Abstract
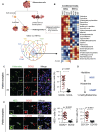
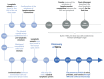
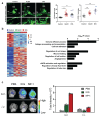
The recent discovery of meningeal lymphatic vessels (MLVs) has revolutionized our understanding of immune regulation within the central nervous system (CNS), overturning the long-standing view of the brain as an immune-privileged organ. Glioblastoma (GBM), the most aggressive primary brain tumor, remains therapeutically intractable due to its highly immunosuppressive microenvironment and poor response to conventional and immune-based therapies. Emerging evidence suggests that MLVs play a crucial role in CNS immune surveillance, cerebrospinal fluid drainage, and solute clearance, all of which are directly linked to GBM pathophysiology. This review is motivated by the urgent need to explore novel therapeutic strategies that address GBM's immune escape and therapeutic resistance. We comprehensively analyze the bidirectional interactions between MLVs and GBM, including their role in antigen transport, T cell activation, and tumor dissemination. Furthermore, we evaluate the therapeutic potential of targeting MLVs through lymphangiogenic stimulation or as alternative routes for immune modulation and drug delivery. These approaches offer promising avenues to enhance anti-tumor immunity and may pave the way for next-generation treatment paradigms in GBM.
Keywords: central nervous system; glioblastoma; glymphatic system; immune modulation; meningeal lymphatic vessels; tumor therapy.
© The author(s).
Conflict of interest statement
Competing interests: The authors have declared that no competing interest exists.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

