Enhancing hair regrowth using rapamycin-primed mesenchymal stem cell-derived exosomes
- PMID: 40585981
- PMCID: PMC12203813
- DOI: 10.7150/thno.107659
Enhancing hair regrowth using rapamycin-primed mesenchymal stem cell-derived exosomes
Abstract
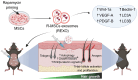
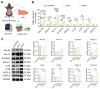
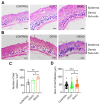
Rationale: Hair loss affects millions globally, with limited effective treatments available and significant psychological impacts. Mesenchymal stem cells (MSCs) and MSC-derived exosomes hold therapeutic potential by modulating cellular communication, reducing inflammation, and supporting hair follicular regeneration. Rapamycin, a mechanistic target of rapamycin (mTOR) inhibitor, enhances MSC therapeutic potential by promoting the release of growth factors and signaling molecules. Thus, this study explores the benefit of priming effect of rapamycin on enhancing the function of MSC-derived exosomes to promote hair regrowth in a depilation-induced murine model. Methods: MSCs were primed with rapamycin, and exosomes were extracted from the MSC-conditioned media using ultrafiltration and poly (ethylene glycol) (PEG) precipitation. Dermal fibroblasts were treated with several doses of exosomes to evaluate the in vitro effect of rapamycin-primed MSC-derived exosomes (REXO). The depilated mice were administered exosomes via intradermal route and the hair regrowth was monitored for 15 days, followed by gene expression analysis and histological examination. Results: Dermal fibroblasts treated with REXO showed a higher proliferation rate and an increase in genes related to Wnt/β-catenin signaling, autophagy, and growth factors compared to non-primed MSC-derived exosomes (CEXO). In vivo REXO therapy via intradermal injection to the depilated areas in mice enhanced hair follicle development, hair density, and hair activation markers compared with the control and naive exosome treatments. Conclusion: REXO therapy effectively enhances hair regrowth thus this approach could offer a clinically effective therapy for hair loss treatment.
Keywords: Wnt/β-catenin signaling; exosome; hair regrowth; mesenchymal stem cell; rapamycin.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

