Endothelium-specific sensing of mechanical signals drives epidermal aging through coordinating retinoid metabolism
- PMID: 40585982
- PMCID: PMC12203922
- DOI: 10.7150/thno.112299
Endothelium-specific sensing of mechanical signals drives epidermal aging through coordinating retinoid metabolism
Abstract
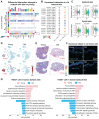
Introduction: Skin aging manifests as a systemic decay of intercellular mechano-chemical coordination. While vascular endothelial cells emerge as central orchestrators, their specific roles in sensing mechanical signals remain poorly understood. Methods: To investigate age-related skin changes, we performed single-cell RNA sequencing (scRNA-seq) and spatial transcriptomics to analyze cellular proportions and differentially expressed genes (DEGs) across young, middle-aged, and elderly human skin samples. The mechanical properties of skin were quantified using photonic crystal cellular force microscopy (PCCFM) to compare Young's modulus between young and aged skin. Cell-cell communication networks, particularly interactions among fibroblasts, vascular endothelial cells, and epidermal cells, were deciphered via CellChat analysis in young versus aged groups. Functional validation of integrin receptors and the MK signaling pathway was conducted using aging mouse models and skin organoid systems. Age-associated biomarkers were identified through immunofluorescence staining, hematoxylin-eosin (HE) staining, and RT-qPCR. RNA-seq further screened downstream targets of the MK pathway. Skin organoid cultures were employed to validate the rejuvenating effects of retinol metabolites. Results: Here we revealed that mechanoresponsive endothelial cells drive skin aging by orchestrating a tripartite axis (fibroblast-endothelial-epidermal) via integrin-mediated mechano-transduction that modulates retinoid metabolism. First, we found that reduced extracellular matrix (ECM) expression by fibroblasts weakens integrin-mediated interactions with endothelial cells, leading to a decreased number of endothelial cells and thinner skin during aging. Then, attenuated endothelial cells-derived MDK signaling to SDC4 in basal cells results in declined basal cell retinol metabolism, a process essential for maintaining skin homeostasis and regeneration. Using our established skin organoid model, we demonstrated that adding retinol metabolites can rejuvenate skin cells with better structural and functional integrity. Conclusions: These findings highlight the intricate intercellular dynamics that underlie skin aging and shed light on the previously underexplored role of mechano-sensitive endothelial cells in this process. Aging as an endothelial-specific coordination failure with other cells in the skin and potentiates developing combinatorial mechano-metabolic intervention strategies to restore tissue-level rejuvenation.
Keywords: aging; endothelial cells; mechano-chemical coordination; retinol metabolism.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures






References
-
- Grunewald M, Kumar S, Sharife H, Volinsky E, Gileles-Hillel A, Licht T. et al. Counteracting age-related VEGF signaling insufficiency promotes healthy aging and extends life span. Science. 2021;373(6554):eabc8479. - PubMed
-
- Franco AC, Aveleira C, Cavadas C. Skin senescence: mechanisms and impact on whole-body aging. Trends Mol Med. 2022;28:97–109. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

