Enhanced neoangiogenesis and balance of the immune response mediated by the Wilms' tumor suppressor WT1 favor repair after myocardial infarction
- PMID: 40585983
- PMCID: PMC12203559
- DOI: 10.7150/thno.104329
Enhanced neoangiogenesis and balance of the immune response mediated by the Wilms' tumor suppressor WT1 favor repair after myocardial infarction
Abstract
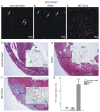
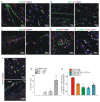
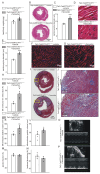
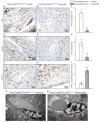
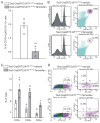
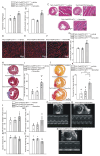
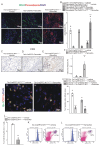
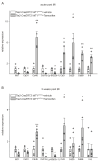
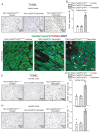
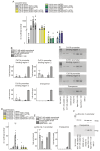
Rationale: Cardiac repair and regeneration are severely constrained in adult mammals. Several cell types have been identified as playing a role in cardiac repair. However, our understanding of the regulatory proteins common to these cell types and implicated in cardiac repair remains limited. Methods: Experimental myocardial infarctions (MI) were induced in mice by ligation of the left coronary artery. WT1 expression in different cell types was determined by immunofluorescent double-labelling. VE-cadherin-CreERT2 (VE-CreERT2) mice were crossed with Wt1lox/lox animals to generate the VE-CreERT2;Wt1lox/lox strain to knockout WT1 in endothelial cells. Wt1lox/lox and Tie2-CreERT2 animals were crossed to generate Tie2-CreERT2;Wt1lox/lox mice to delete WT1 in endothelial and myeloid-derived cells. Results: We show that the Wilms' tumor suppressor WT1 is expressed in progenitor cell populations, endothelial cells, and myeloid-derived suppressor cells (MDSCs) in mice following MI. Endothelial-specific knockout of WT1 results in reduced vascular density after MI but does not affect functional recovery. Conversely, combined knockout of WT1 in endothelial and myeloid-derived cells increases infarct size, cardiac hypertrophy, fibrosis, hypoxia, and lymphocyte infiltration. Notably, angiogenesis, infiltration of MDSCs, and cellular proliferation were diminished, and importantly, cardiac function was reduced. Mechanistically, in addition to the previously established role of WT1 in promoting the expression of angiogenic molecules, this transcription factor positively regulates the expression of Cd11b and Ly6G, which are crucial for MDSC invasion, migration and function thereby preventing overactivation of the immune response. Conclusions: Several molecules have been identified that are implicated in distinct aspects of cardiac repair following MI. The identification of WT1 as a transcription factor that is essential for repair mechanisms involving various cell types within the heart may potentially enable the future development of a coordinated repair process following myocardial infarction.
Keywords: Wilms' tumor suppressor; angiogenesis; cardiac repair; immune response; myocardial infarction.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
Mitochondrial Tumor Suppressor 1A Attenuates Myocardial Infarction Injury by Maintaining the Coupling Between Mitochondria and Endoplasmic Reticulum.Circulation. 2025 Jul 22;152(3):183-201. doi: 10.1161/CIRCULATIONAHA.124.069737. Epub 2025 Jun 30. Circulation. 2025. PMID: 40583767
-
PMN-MDSCs are responsible for immune suppression in anti-PD-1 treated TAP1 defective melanoma.Clin Transl Oncol. 2025 Jul;27(7):3073-3083. doi: 10.1007/s12094-024-03840-7. Epub 2025 Jan 18. Clin Transl Oncol. 2025. PMID: 39825997
-
Sipa1 Drives a Maladaptive Fibroblast-Myeloid Axis After Myocardial Infarction.Circ Res. 2025 Aug;137(4):533-547. doi: 10.1161/CIRCRESAHA.124.326030. Epub 2025 Jun 26. Circ Res. 2025. PMID: 40567222
-
Exercise-based rehabilitation for coronary heart disease.Cochrane Database Syst Rev. 2000;(4):CD001800. doi: 10.1002/14651858.CD001800. Cochrane Database Syst Rev. 2000. Update in: Cochrane Database Syst Rev. 2001;(1):CD001800. doi: 10.1002/14651858.CD001800. PMID: 11034729 Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Cahill TJ, Choudhury RP, Riley PR. Heart regeneration and repair after myocardial infarction: translational opportunities for novel therapeutics. Nat Rev Drug Discov. 2017;16:699–717. - PubMed
-
- González-Rosa JM, Mercader N. Cryoinjury as a myocardial infarction model for the study of cardiac regeneration in the zebrafish. Nat Protoc. 2012;7:782–788. - PubMed
-
- Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

