Combination of KRAS ASO and RIG-I agonist in extracellular vesicles transforms the tumor microenvironment towards effective treatment of KRAS-dependent cancers
- PMID: 40585984
- PMCID: PMC12203666
- DOI: 10.7150/thno.105519
Combination of KRAS ASO and RIG-I agonist in extracellular vesicles transforms the tumor microenvironment towards effective treatment of KRAS-dependent cancers
Abstract
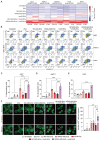
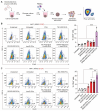
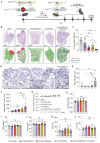
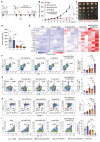
Rationale: Mutations in the KRAS gene drive many cancers, yet targeting KRAS mutants remains a challenge. Here, we address this hurdle by utilizing a nucleic acid-based therapeutic strategy delivered via extracellular vesicles (EVs) to simultaneously inhibit KRAS mutants and activate the RIG-I pathway, aiming to enhance anti-tumor immunity. Methods: Antisense oligonucleotides against KRAS mutants (KRAS ASOs) and RIG-I agonist immunomodulatory RNA (immRNA) were loaded into EVs and administered to KRAS-mutant cancer models. The therapeutic effects were assessed in colorectal and non-small cell lung cancer (NSCLC) tumor models, as well as patient-derived pancreatic cancer organoids. Immune responses were evaluated by analyzing tumor microenvironment's changes, dendritic cell activation, and T cell memory formation. The treatment efficacy was evaluated based on the tumor development and overall survival. Results: The KRAS-ASO and immRNA combination treatment induced immunogenic tumor cell death and upregulated interferons in KRAS-dependent cancers. In a colorectal tumor model, the therapy shifted the tumor microenvironment to an immunogenic state, activated dendritic cells in sentinel lymph nodes, and promoted memory T cell formation. In an aggressive NSCLC model, the treatment resulted in a strong anti-tumor activity and extended survival without any adverse effects. Validation in patient-derived pancreatic cancer organoids confirmed the clinical translation potential of this approach. Conclusions: EV-mediated delivery of ASOs and immRNA effectively inhibits KRAS mutants and activates RIG-I, leading to a robust anti-tumor immune response. This strategy holds promise for effectively treating KRAS-driven cancers and improving clinical outcomes.
Keywords: KRAS mutation; RIG-I agonist; antisense oligonucleotides; cancer therapy; extracellular vesicles.
© The author(s).
Conflict of interest statement
Competing Interests: Minh T.N Le is a cofounder and advisor of Carmine Therapeutics, which develops gene therapy and has a patent relating to Vesicle-Based Compositions and Uses Thereof. Carmine Therapeutics provides a proprietary transfection reagent required for loading of ASOs into EVs used in this study. C.D. Phung, B. Peng, T.M. Nguyen, and D. Luo have a patent relating to Vesicle-Based Compositions and Uses Thereof. Other authors declare no competing financial interest.
Figures


References
-
- Buscail L, Bournet B, Cordelier P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2020;17:153–68. - PubMed
-
- Jonckheere N, Vasseur R, Van Seuningen I. The cornerstone K-RAS mutation in pancreatic adenocarcinoma: From cell signaling network, target genes, biological processes to therapeutic targeting. Crit Rev Oncol Hematol. 2017;111:7–19. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

