A subset of evolutionarily conserved centriolar satellite core components is crucial for sperm flagellum biogenesis
- PMID: 40585997
- PMCID: PMC12203921
- DOI: 10.7150/thno.117118
A subset of evolutionarily conserved centriolar satellite core components is crucial for sperm flagellum biogenesis
Abstract
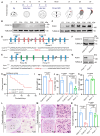
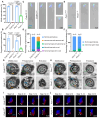
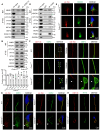
Rationale: Centriolar satellites are non-membranous cytoplasmic granules that cluster around centrosomes, with pericentriolar material 1 (PCM1) serving as the molecular marker for these structures. Although significant progress has been made in understanding their composition, cellular, and organismal functions over the past decades, the tissue-specific roles of centriolar satellite proteins in sperm flagellum biogenesis and male fertility are still not well understood. Methods: We utilize publicly available data and conduct phylogenetic analysis to explore the tissue distribution and conservation of centriole satellite components across flagellated species. Knockout mouse models for Ccdc13 and Pcm1 were constructed to investigate their physiological roles. Sperm morphology and functionality were analyzed using immunofluorescence, transmission electron microscopy, and sperm motility analysis. Immunofluorescence, immunoblotting, co-immunoprecipitation, and proteomics analyses were carried out to elucidate the molecular mechanisms by which CCDC13 regulates sperm flagellum biogenesis. Results: We show that most satellite components are expressed in the testis and associated with ciliary function. Comparative analysis of ciliary-related satellite components across 11 flagellated and non-flagellated species revealed six highly conserved satellite proteins in flagellated species. PCM1, a well-known centriolar satellite scaffolding protein, was found to be less conserved. Based on these findings, we selected CCDC13, a highly conserved satellite protein, and PCM1, a less conserved component, for functional comparison in sperm flagellum biogenesis. Using knockout mouse models, we demonstrated that Ccdc13 deficiency led to male infertility with multiple morphological abnormalities of the sperm flagella (MMAF)-like phenotype due to defects in sperm flagellum biogenesis. While Pcm1 knockout only resulted in decreased sperm motility without affecting flagellum biogenesis. Molecularly, CCDC13 interacts with IMT, IFT-associated proteins, and flagellar components to regulate transport of cargo to proper positions for flagellum biogenesis. Conclusion: This study identifies a subset of highly conserved centriolar satellite proteins essential for sperm flagellum biogenesis. The identification of these proteins provides valuable insights into the genetic mechanisms underlying flagellum function and their evolutionary development. Additionally, defects in these proteins may be associated with male infertility in humans.
Keywords: CCDC13; PCM1; centriolar satellites; flagellum biogenesis; male infertility.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




Similar articles
-
Loss of C-Terminal Coiled-Coil Domains in SDCCAG8 Impairs Centriolar Satellites and Causes Defective Sperm Flagellum Biogenesis and Male Fertility.Cells. 2025 Jul 23;14(15):1135. doi: 10.3390/cells14151135. Cells. 2025. PMID: 40801568 Free PMC article.
-
TonEBP inhibits ciliogenesis by controlling aurora kinase A and regulating centriolar satellite integrity.Cell Commun Signal. 2024 Jul 3;22(1):348. doi: 10.1186/s12964-024-01721-8. Cell Commun Signal. 2024. PMID: 38961488 Free PMC article.
-
CCDC89 is required for optimal sperm motility and male fertility in mammals.Hum Reprod. 2025 Sep 1;40(9):1616-1628. doi: 10.1093/humrep/deaf126. Hum Reprod. 2025. PMID: 40591933
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
Cited by
-
Loss of C-Terminal Coiled-Coil Domains in SDCCAG8 Impairs Centriolar Satellites and Causes Defective Sperm Flagellum Biogenesis and Male Fertility.Cells. 2025 Jul 23;14(15):1135. doi: 10.3390/cells14151135. Cells. 2025. PMID: 40801568 Free PMC article.
References
-
- Satir P, Mitchell DR, Jékely G. How did the cilium evolve? Curr Top Dev Biol. 2008;85:63–82. - PubMed
-
- Takeda S, Narita K. Structure and function of vertebrate cilia, towards a new taxonomy. Differentiation. 2012;83:S4–11. - PubMed
-
- Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

