Dataset of Fourier transform infrared (FTIR) spectra of a primary amine, selected aldehydes, and their Schiff bases acquired using the Invenio-R spectrometer
- PMID: 40586092
- PMCID: PMC12205674
- DOI: 10.1016/j.dib.2025.111741
Dataset of Fourier transform infrared (FTIR) spectra of a primary amine, selected aldehydes, and their Schiff bases acquired using the Invenio-R spectrometer
Abstract
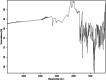
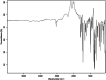
This dataset reports on the Fourier-transform infrared (FTIR) spectroscopic analysis of a primary amine and some aldehydes along their corresponding Schiff bases. The dataset presented in this work consists of spectral FTIR data acquired in the 80-6000 cm⁻¹ range, along with the corresponding relative IR intensities of the samples. The primary amine and aldehydes were of high purity and were used to synthesise the corresponding Schiff bases. The FTIR spectra were acquired for the vibrational assignment associated with the samples. The IR spectra were acquired using a Bruker Invenio-R (Universiti Putra Malaysia, Malaysia) spectrometer equipped with attenuated total reflection (ATR) diamond crystal with an accumulation of 64 scans at a spectral resolution of 4 cm⁻¹ and processed using OPUS 8.7.41 software. The data indicated that FTIR spectroscopy can be a powerful tool for analysing Schiff bases.
Keywords: ATR-FTIR; Aldehydes; Imines; Primary amine; Schiff base.
© 2025 The Author(s).
Figures
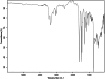



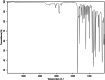
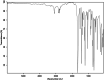

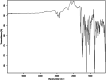



References
-
- Sehrawat S., Sandhu N., Anand V., Pandey S.K., Sharma A., Yadav R.K., Singh A.P., Singh A.P. Study of 5-Bromo-2-thiophene carboxaldehyde derived novel Schiff base as a biologically active agent as well as X-ray crystallographic study of C-S coupled benzothiazole. J. Mol. Struct. 2022;1269:133782. doi: 10.1016/j.molstruc.2022.133782. - DOI
-
- Dllmaghani K.A., Jazani N.H., Behrouz A., Fakhraee F.M. Synthesis, characterization and antibacterial activity of some schiff bases derived from 4-aminobenzoic acid. Asian J. Chem. 2009;21:5947–5954. https://www.scopus.com/inward/record.uri?eid=2-s2.0-69749110127&partnerI...
-
- Ourari A., Khelafi M., Aggoun D., Bouet G., Khan M.A. Synthesis, characterization, and electrochemical study of tetradentate ruthenium-schiff base complexes: Dioxygen activation with a cytochrome P450 model using 1-or 2-methylimidazole as axial bases. Adv. Phys. Chem. 2011;(2011) doi: 10.1155/2011/157484. - DOI
-
- Devidas S.M., Quadri S.H., Kamble S.A., Syed F.M., Vyavhare D.Y. Novel one-pot synthesis of schiff base compounds derived from different diamine & aromatic aldehyde catalyzed by P 2O 5/SiO 2 under free-solvent condition at room temperature. J. Chem. Pharm. Res. 2011;3:489–495. https://www.scopus.com/inward/record.uri?eid=2-s2.0-79954455646&partnerI...
-
- Dal H. Intramolecular hydrogen bonding and tautomerism in schiff bases: synthesis, spectroscopic and theoretical studies of n-(2,2’-methylenebis(4-methoxyphenyl) salicylidene and n-(2,2’-methylenebis(4-methoxyphenyl)-2-oxonaphthalidene- methylamine. Asian J. Chem. 2014;26:2759–2767. doi: 10.14233/ajchem.2014.16725. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

