Electroencephalogram features support the retrogenesis hypothesis of Alzheimer's disease: Exploratory comparison of brain changes in aging and childhood
- PMID: 40586221
- PMCID: PMC12322342
- DOI: 10.1177/13872877251352119
Electroencephalogram features support the retrogenesis hypothesis of Alzheimer's disease: Exploratory comparison of brain changes in aging and childhood
Abstract
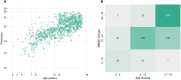
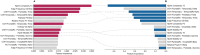
BackgroundThe retrogenesis hypothesis (RH) suggests that the functional and cognitive decline observed in Alzheimer's disease dementia mirrors in reverse order the brain development during childhood and adolescence.ObjectiveEquivalent electroencephalogram (EEG) patterns between older adults across different cognitive decline stages and children across different brain maturation stages were directly compared.MethodsTo capture the complex patterns that allow for such a comparison, a regression model was trained on EEG data from N = 510 older adults, at different stages of cognitive reserve, to identify EEG markers predictive of global cognitive status. The model was then applied on the same EEG markers of N = 696 children across different ages.ResultsThe model predicted MMSE scores with an average error of 2.53 and R2 of 0.80. When applied to children, predictions correlated positively with age (r = 0.73). Key predictors of cognitive function concordant in both populations were theta coherence (right frontal-left temporal/parietal), temporal Hjorth complexity, and beta edge frequency, supporting the RH.ConclusionsThese EEG features were inversely associated between older adults and children, supporting a functional underpinning of the retrogenesis model of dementia. Clinical validation of these biomarkers could favor their use in the continuous monitoring of cognitive function.
Keywords: Alzheimer's disease; Hjorth complexity; Mini-Mental State Exam; cognitive function; developmental age; electroencephalography; frontotemporal dementia; machine learning; spectral coherence.
Conflict of interest statement
Declaration of conflicting interestsThe authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S.T. was member of advisory boards for Lilly, Eisai, Biogen, and GE Healthcare. He is member of the Independent Data Safety and Monitoring Board of the study ENVISION (Biogen). H.H. is an employee of Eisai Inc.; however, this article does not represent the opinion of Eisai. H.H. declares no competing financial interests related to the present article, and his contribution to this article reflects only and exclusively his academic and scientific expertise as part of an academic appointment at Sorbonne University, Paris, France. He serves as a Reviewing Editor and previously as Senior Associate Editor for the journal Alzheimer's & Dementia. Part of this study was initiated and developed in line with the Alzheimer's Precision Medicine Initiative (APMI) and Neurodegeneration Precision Medicine (NPMI) framework. The remaining authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures





References
-
- Reisberg B, Franssen EH, Hasan SM, et al. Retrogenesis: clinical, physiologic, and pathologic mechanisms in brain aging, Alzheimer’s and other dementing processes. Eur Arch Psychiatry Clin Neurosci 1999; 249: S28–S36. - PubMed
-
- Reisberg B, Ferris SH, Anand R, et al. Functional staging of dementia of the Alzheimer type. Ann N Y Acad Sci 1984; 435: 481–483.
-
- Gayraud F, Loureiro IS, Frouin C, et al. Lexical deterioration in Alzheimer’s disease. In: 10th International Conference of Experimental Linguistics, Lisbonne, Portugal, pp. 101–104.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

