Effectiveness of an Autologous Micrografting Technology for Treating Stretch Marks
- PMID: 40586239
- PMCID: PMC12207717
- DOI: 10.1111/jocd.70321
Effectiveness of an Autologous Micrografting Technology for Treating Stretch Marks
Abstract
Background: Stretch marks or striae distensae (SD) are common dermal lesions caused by the disruption of collagen and elastin fibers in the skin, often triggered by rapid mechanical stretching. Despite the availability of numerous treatment modalities, from topical agents to energy-based devices, no single therapy has demonstrated consistent, long-term efficacy across all patient populations. The pathophysiology of SD involves complex alterations in the extracellular matrix (ECM), particularly affecting fibroblast activity and collagen/elastin synthesis.
Aims: This pilot study aims to evaluate the clinical and molecular efficacy of autologous micrografting technology as a novel therapeutic option for SD. Specifically, it investigates the treatment's impact on ECM-related gene expression and overall skin appearance.
Patients/methods: Fourteen patients (13 females, 1 male) with clinically evident SD were enrolled. All participants underwent a standardized treatment protocol comprising microneedling followed by intradermal injection of autologous micrografts, obtained via a minimally invasive procedure. Clinical assessments were performed through standardized photography at baseline, 1 month, and 6 months post-treatment. In vitro assays were conducted on cultured human dermal fibroblasts exposed to the micrograft suspension.
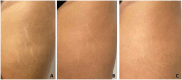
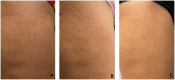
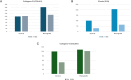
Results: Clinical evaluations showed noticeable aesthetic improvements, including reduced striae visibility and improved skin texture, with high patient-reported satisfaction. Molecular analyses revealed the upregulation of key ECM genes, including COL4A1, COL6A1, and ELN, indicating enhanced fibroblast activation and regenerative potential.
Conclusions: Autologous micrografting appears to be a promising, biologically active approach for SD treatment. It promotes ECM remodeling by stimulating fibroblast function and may represent a valuable addition to the therapeutic landscape for stretch marks.
Keywords: autologous micrografts; collagen; elastin; gene expression; stretch marks.
© 2025 The Author(s). Journal of Cosmetic Dermatology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Al‐Himdani S., Ud‐Din S., Gilmore S., and Bayat A., “Striae Distensae: A Comprehensive Review and Evidence‐Based Evaluation of Prophylaxis and Treatment,” British Journal of Dermatology 170, no. 3 (2014): 527–547. - PubMed
-
- Maari C. and Powell J., “Atrophies of Connective Tissue,” in Dermatology, 4th ed., ed. Bolognia J. L., Schaffer J. V., and Cerroni L. (Elsevier, 2003), 1723–1732.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

