Chemically Recyclable Polyester Thermosets from Activated Adipic Acid and Renewable Polyols
- PMID: 40586292
- PMCID: PMC12487748
- DOI: 10.1002/cssc.202500880
Chemically Recyclable Polyester Thermosets from Activated Adipic Acid and Renewable Polyols
Abstract
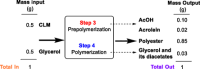
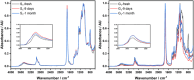
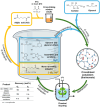
This study outlines a method for producing chemically recyclable crosslinked polyesters using renewable polyols-glycerol and sorbitol-combined with adipic acid (AA), which is transformed/activated into a polyanhydride mixture prior to use. A three-step procedure has been designed: 1) an acid-catalyzed reaction of AA with nontoxic isopropenyl acetate or acetic anhydride to form a crosslinking mixture (CLM) made of adipic-acetic mixed polyanhydrides; 2) a solvent- and additive-free process where glycerol or sorbitol, or a combination thereof, is reacted with the CLM to achieve a prepolymer, and 3) a casting/molding of the liquid viscous prepolymer to yield a thermoset as the end product. Different thermosets (eight examples) are prepared by changing the reagents ratio. These solids are thoroughly characterized by tensile tests, DMA, high-resolution magic angle spinning and solid-state NMR, thermal gravimetric analysis, DSC, and fourier transformed infra red (FT-IR) spectroscopy. The formation of cross-linked polyesters is confirmed in all cases, but mechanical properties varied significantly from one specimen to another. Interestingly, a tensile strength up to 18 MPa-approximately an order of magnitude higher than similar polymers-is achieved when sorbitol and the CLM are used in a 1:1 wt% ratio. The chemical recycle of the resulting polymers is achieved via methanolysis with quantitative recovery of the monomeric units.
Keywords: bioplastics; chemical recycling; isopropenyl acetate; solvent‐free; upcycling.
© 2025 The Author(s). ChemSusChem published by Wiley‐VCH GmbH.
Conflict of interest statement
Davide Rigo, Maurizio Selva, Alvise Perosa and Matteo Lorenzon have filed a patent application on this concept (patent number: IT102025000006018).
Figures
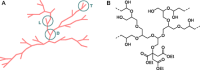

 Symmetric anhydrides
Symmetric anhydrides  Acetyl anhydrides
Acetyl anhydrides  Isopropenyl esters
Isopropenyl esters  Adipic anhydride (1). Determined by 1H NMR with dioxane as the internal standard. The decline in isopropenyl ester signals was due to the formation of mixed anhydrides via an acid‐catalyzed transesterification with carboxylic acid groups, accompanied by the release of acetone.
Adipic anhydride (1). Determined by 1H NMR with dioxane as the internal standard. The decline in isopropenyl ester signals was due to the formation of mixed anhydrides via an acid‐catalyzed transesterification with carboxylic acid groups, accompanied by the release of acetone.


References
-
- Chinthapalli R., Skoczinski P., Carus M., Baltus W., De Guzman D., Käb H., Raschka A., Ravenstijn J., Ind. Biotechnol. 2019, 15, 237.
-
- Walker S., Rothman R., J. Clean. Prod. 2020, 261, 121158.
-
- Zhu Y., Romain C., Williams C. K., Nature 2016, 540, 354. - PubMed
-
- Ritzen L., Sprecher B., Bakker C., Balkenende R., Resour. Conserv. Recycl. 2023, 199, 107268.
-
- Wilbon P. A., Chu F., Tang C., Macromol. Rapid. Commun. 2013, 34, 8. - PubMed
LinkOut - more resources
Full Text Sources

