Association of a giant developmental venous anomaly and acute disseminated encephalomyelitis: A case report and magnetic resonance perfusion study
- PMID: 40586451
- PMCID: PMC12209250
- DOI: 10.1177/19714009251356989
Association of a giant developmental venous anomaly and acute disseminated encephalomyelitis: A case report and magnetic resonance perfusion study
Abstract
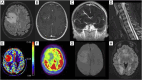
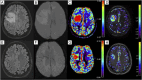
We describe the first reported association between acute disseminated encephalomyelitis (ADEM) and giant developmental venous anomaly (DVA) in the context of myelin oligodendrocyte associated glycoprotein (MOG) associated disorder (MOGAD). Patient was a young woman presenting with headache, bradypsychia and tetrapyramidal syndrome. Imaging showed disseminated tumefactive inflammatory lesions in the brain and spinal cord, with a massive right frontal lobe lesion centred around a giant DVA. Demyelinating inflammatory lesions are known to occur in a perivenular pattern, and the association between some inflammatory diseases such as multiple sclerosis (MS) and DVA has already been described. Developmental venous anomalies are variant of the normal venous drainage of the brain, responsible of a local alteration of the venular network, and micro-perfusion anomalies as well as possible increased of blood-brain barrier permeability. As such, they might be responsible for a favourable environment for pathogenic auto-antibodies penetrance in such region, potentializing the inflammatory lesion size. Perfusion imaging showed a significant increase in regional blood volume and blood transit time in the DVA and the surrounding brain tissue, which regressed in the follow-up imaging studies after the acute stage. This case illustrates the potential role of DVA in the setting of demyelinating diseases, and its consequences on the local micro-perfusion of the brain, evolving between the acute and chronic phase of the illness.
Keywords: ADEM; MOGAD; demyelination; developmental venous anomaly; perfusion imaging.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
-
- Rammos SK, Maina R, Lanzino G. Developmental venous anomalies: current concepts and implications for management. Neurosurgery 2009; 65(1): 20–29. - PubMed
-
- San Millán Ruíz D, Gailloud P. Cerebral developmental venous anomalies. Childs Nerv Syst 2010; 26(10): 1395–1406. - PubMed
-
- Nagatani K, Osada H, Takeuchi S, et al. Surgical resection of developmental venous anomaly causing massive intracerebral haemorrhage: a case report. Br J Neurosurg 2014; 28(1): 116–118. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

