Genetic resilience or resistance in poultry against avian influenza virus: mirage or reality?
- PMID: 40586559
- PMCID: PMC12282110
- DOI: 10.1128/jvi.00820-25
Genetic resilience or resistance in poultry against avian influenza virus: mirage or reality?
Abstract
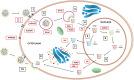
The unprecedented global spread of the highly pathogenic avian influenza (HPAI) virus in wild birds, poultry, and mammalian species has challenged our control efforts. Alternative approaches to limit avian influenza viruses (AIV) include the development of resilient or resistant chickens. Genetically resilient birds may become infected but can overcome disease, whereas resistant birds prevent virus attachment or entry and do not become infected. The most intensively studied host gene is myxovirus-resistance (Mx), which is expressed via the interferon pathway. Both sensitive and resistant chicken Mx genotypes have been described, but this only provides limited resilience. Acidic nuclear phosphoprotein 32 family member A (ANP32A) has been demonstrated as a host cofactor for AIV replication via interaction with the polymerase. Small nucleotide changes within this gene have demonstrated some promise for the establishment of disease resilience. Certain MHC-defined genetic chicken lines have demonstrated increased resilience with higher innate immune responses, but HPAI-infected birds still have high morbidity and mortality. Alternatively, gene-edited or -transgenic chickens have had some success in increasing resilience. This strategy allows flexibility to include foreign genes, modification of existing genes, or combined approaches to block critical steps in the viral life cycle. Some candidate genes include solute carrier 35A1 (SLC35A1), retinoic acid-inducible gene I (RIG-I), and toll-like receptors 3 and 7 (TLR3/7), but animal testing needs to be conducted. Furthermore, existing hurdles for technology transfer to commercial application from either naturally occurring resistance genes or foreign genes remain high and will require acceptance by both the poultry industry and consumers.
Keywords: MHC haplotype; chicken; influenza; interferon; resilience; resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Suarez DL. 2016. Influenza a virus, p 1–30. In Swayne DE (ed), Animal influenza. John Wiley & Sons, Inc.
-
- Swayne DE, Pantin-Jackwood M. 2006. Pathogenicity of avian influenza viruses in poultry. Dev Biol (Basel) 124:61–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

