Anomalous Water Fluorescence Induced by Solutes
- PMID: 40586586
- PMCID: PMC12257589
- DOI: 10.1021/acs.jpclett.5c01189
Anomalous Water Fluorescence Induced by Solutes
Abstract
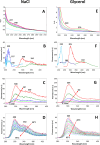
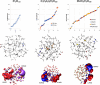
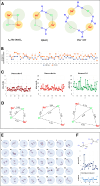
The origin of recently reported anomalous fluorescence emissions from aqueous solutions of nonaromatic solutes remains elusive. To determine whether the solute nature influences the fluorescence characteristics and to identify a potential common mechanism, we measured the fluorescence spectra of 21 different solutions. We observed similar emission characteristics across all samples, suggesting that the solute nature plays a minimal role in the emission mechanism. Using time-dependent density functional theory on large water, NaCl/water, and glycerol/water clusters, we attributed the anomalous emission to the decay of charge-transfer-to-solvent excitations (CTTS) which populate a diradical zwitterionic excited state localized at hydrogen-bond network defects. The Arrhenius-like plots for NaCl and glycerol solutions revealed that the S1 nonradiative decay pathway involves the diradical recombination via librational motion. We propose that the presence of solute molecules slows this process, thus increasing the lifetime of the CTTS excited states and facilitating emission.
Figures


Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Larvivorous fish for preventing malaria transmission.Cochrane Database Syst Rev. 2017 Dec 11;12(12):CD008090. doi: 10.1002/14651858.CD008090.pub3. Cochrane Database Syst Rev. 2017. PMID: 29226959 Free PMC article.
-
Larvivorous fish for preventing malaria transmission.Cochrane Database Syst Rev. 2013 Dec 10;(12):CD008090. doi: 10.1002/14651858.CD008090.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2017 Dec 11;12:CD008090. doi: 10.1002/14651858.CD008090.pub3. PMID: 24323308 Free PMC article. Updated.
References
-
- Huang C., Wikfeldt K. T., Tokushima T., Nordlund D., Harada Y., Bergmann U., Niebuhr M., Weiss T. M., Horikawa Y., Leetmaa M., Ljungberg M. P., Takahashi O., Lenz A., Ojamäe L., Lyubartsev A. P., Shin S., Pettersson L. G. M., Nilsson A.. The Inhomogeneous Structure of Water at Ambient Conditions. Proc. Natl. Acad. Sci. U. S. A. 2009;106(36):15214–15218. doi: 10.1073/pnas.0904743106. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

