B7-H3 CAR T Cells Are Effective against Ependymomas but Limited by Tumor Size and Immune Response
- PMID: 40586675
- PMCID: PMC12402791
- DOI: 10.1158/1078-0432.CCR-24-3083
B7-H3 CAR T Cells Are Effective against Ependymomas but Limited by Tumor Size and Immune Response
Abstract
Purpose: Targeted treatments are desperately needed for ependymomas. Chimeric antigen receptor (CAR) T cells have immense potential to transform patient outcomes. However, CAR T-cell therapy for ependymomas has been largely understudied. In this study, we explore the potential of targeting B7 homolog 3 (B7-H3/CD276) with CAR T cells to treat pediatric ependymomas.
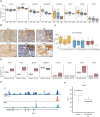
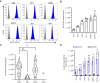
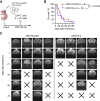
Experimental design: We profiled B7-H3 protein expression in 44 pediatric ependymoma samples by IHC. We generated second-generation human B7-H3.CAR T cells and examined their anti-ependymoma activity in six in vitro and two in vivo xenograft models. We validated findings using HER2-targeted CAR T cells. In addition, we used murine B7-H3.CAR T cells to evaluate in vivo antitumor activity in a fully syngeneic supratentorial ependymoma model.
Results: The majority of clinical ependymoma samples (29/44) stained positive for B7-H3, indicating high but heterogeneous expression across patients. In vitro, human B7-H3.CAR T cells had potent anti-ependymoma cytolytic activity, expansion, and persistence, which was inversely correlated with the upregulation of B7-H3 on CAR T cells. We found that CAR T cells favor type 2 cytokines phenotypes after repeated exposure to ependymoma cell lines, which may be driven by C-C motif chemokine ligand 2 secretion by ependymomas. In vivo, there was potent and significant antitumor activity in human xenograft ependymoma models. However, response durability was limited and significantly correlated with the degree of tumor burden. In the syngeneic setting, murine B7-H3.CAR T-cell efficacy against ependymomas was limited and did not extend survival.
Conclusions: Our results support ongoing clinical evaluation of B7-H3.CAR T cells for ependymomas and provide model systems for further studying determinants of anti-ependymoma CAR T-cell treatment efficacy and resistance.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
G. Krenciute reports grants from the National Institute of Neurological Disorders and Stroke and the NCI during the conduct of the study. No disclosures were reported by the other authors.
Figures



References
-
- Saleh AH, Samuel N, Juraschka K, Saleh MH, Taylor MD, Fehlings MG. The biology of ependymomas and emerging novel therapies. Nat Rev Cancer 2022;22:208–22. - PubMed
-
- Morrall M, Reed-Berendt R, Moss K, Stocks H, Houston AL, Siddell P, et al. Neurocognitive, academic and functional outcomes in survivors of infant ependymoma (UKCCSG CNS 9204). Childs Nerv Syst 2019;35:411–20. - PubMed
-
- Ritzmann TA, Rogers HA, Paine SML, Storer LCD, Jacques TS, Chapman RJ, et al. A retrospective analysis of recurrent pediatric ependymoma reveals extremely poor survival and ineffectiveness of current treatments across central nervous system locations and molecular subgroups. Pediatr Blood Cancer 2020;67:e28426. - PubMed
MeSH terms
Substances
Grants and funding
- Collaborative Ependymoma Research Network
- Alex's Lemonade Stand Foundation for Childhood Cancer (ALSF)
- R01NS121249/National Institute of Neurological Disorders and Stroke (NINDS)
- CA220510/U.S. Department of Defense (DOD)
- P30 CA021765/CA/NCI NIH HHS/United States
- CA220247/U.S. Department of Defense (DOD)
- St. Jude Children's Research Hospital Research Collaborative on Transcription Regulation in Pediatric Cancer Grant
- U01CA281823/National Cancer Institute (NCI)
- R01 NS121249/NS/NINDS NIH HHS/United States
- R01 NS122859/NS/NINDS NIH HHS/United States
- R01 CA284455/CA/NCI NIH HHS/United States
- R01NS122859/National Institute of Neurological Disorders and Stroke (NINDS)
- American Lebanese Syrian Associated Charities (ALSAC)
- P30CA021765/National Cancer Institute (NCI)
- R01CA284455/National Cancer Institute (NCI)
- R01 CA280203/CA/NCI NIH HHS/United States
- R01CA280203/National Cancer Institute (NCI)
- National Brain Tumor Society (NBTS)
- U01 CA281823/CA/NCI NIH HHS/United States
- Assisi Foundation of Memphis (AFM)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

