Evolution of lateralized gustation in nematodes
- PMID: 40586702
- PMCID: PMC12208668
- DOI: 10.7554/eLife.103796
Evolution of lateralized gustation in nematodes
Abstract
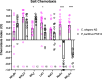
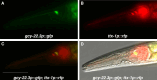
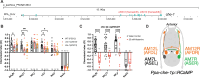
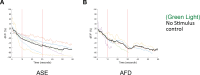
Animals with small nervous systems have a limited number of sensory neurons that must encode information from a changing environment. This problem is particularly exacerbated in nematodes that populate a wide variety of distinct ecological niches but only have a few sensory neurons available to encode multiple modalities. How does sensory diversity prevail within this constraint in neuron number? To identify the genetic basis for patterning different nervous systems, we demonstrate that sensory neurons in Pristionchus pacificus respond to various salt sensory cues in a manner that is partially distinct from that of the distantly related nematode Caenorhabditis elegans. Previously we showed that P. pacificus likely lacked bilateral asymmetry (Hong et al., 2019). Here, we show that by visualizing neuronal activity patterns, contrary to previous expectations based on its genome sequence, the salt responses of P. pacificus are encoded in a left/right asymmetric manner in the bilateral ASE neuron pair. Our study illustrates patterns of evolutionary stability and change in the gustatory system of nematodes.
Keywords: Pristionchus pacificus; calcium imaging; developmental biology; lateral asymmetry.
© 2025, Mackie et al.
Conflict of interest statement
MM, VL, HC, NK, DC, ID, KQ, SC, OH, SC, RH No competing interests declared
Figures





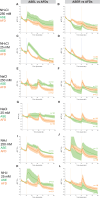

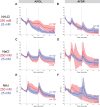

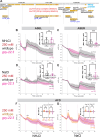


Update of
-
Evolution of lateralized gustation in nematodes.bioRxiv [Preprint]. 2025 Mar 6:2024.08.31.610597. doi: 10.1101/2024.08.31.610597. bioRxiv. 2025. Update in: Elife. 2025 Jun 30;14:RP103796. doi: 10.7554/eLife.103796. PMID: 39282255 Free PMC article. Updated. Preprint.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

