Propofol Modulates Microglial Glucose Metabolism Via the AMPK/HIF-1α Signaling Pathway To Ameliorate ECS-induced Cognitive Deficits in Depressive-like Rats
- PMID: 40587030
- PMCID: PMC12209389
- DOI: 10.1007/s11064-025-04473-0
Propofol Modulates Microglial Glucose Metabolism Via the AMPK/HIF-1α Signaling Pathway To Ameliorate ECS-induced Cognitive Deficits in Depressive-like Rats
Abstract
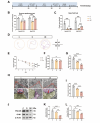
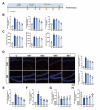
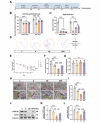
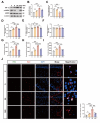
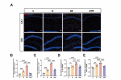
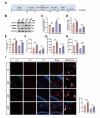
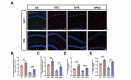
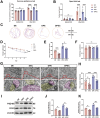
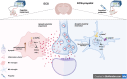
Propofol can partly ameliorate electroconvulsive shock (ECS)-induced learning and memory impairment by restoring synaptic plasticity. However, the exact mechanism is unknown. Microglia exert different immune functions by regulating their glucose metabolism, which is closely related to synaptic plasticity. We aimed to investigate whether the mechanism underlying the cognitive enhancement effects of propofol is associated with microglial glucose metabolism. Rats depression model was established by chronic unpredictable mild stress (CUMS). Sucrose preference test (SPT) and open field test (OFT) were used to detect anhedonia and anxiety-like behaviors in rats, respectively. Morris water maze (MWM) was used to evaluate the spatial learning and memory ability of rats. Transmission electron microscopy, immunofluorescence, enzymatic activity assays, Western blotting, and RT-qPCR were employed to evaluate hippocampal synaptic structural integrity, microglial glucose metabolism, and the expression of glycolytic regulators p-AMPK/AMPK and HIF-1α. The AMPK inhibitor compound C was used for reverse validation. Propofol attenuated the ECS-induced reduction of hippocampal synaptic proteins PSD-95 and SYN1, suppressed the upregulation of pro-inflammatory cytokines TNF-α and IL-1β, and reduced microglial activation. It also reduced the key glycolytic enzymes in microglia, increased AMPK expression, and decreased HIF-1α expression, thereby improving learning and memory impairment in ECS-treated rats. Compound C reversed propofol's neuroprotective effect. ECS-induced learning and memory deficits in depressive-like rats are associated with increased microglial glycolysis via the AMPK/HIF-1α pathway, a metabolism process that could be mitigated by propofol.
Keywords: Cognitive Impairment; Depression; Electroconvulsive Shock; Metabolic Reprogramming; Microglia; Propofol.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics Approval: All studies received ethical approval from the Ethics Committee of Chongqing Medical University (Chongqing, China). Competing Interests: The authors declare no competing interests.
Figures
Similar articles
-
Propofol improves sleep deprivation-induced sleep structural and cognitive deficits via upregulating the BMAL1 expression and suppressing microglial M1 polarization.CNS Neurosci Ther. 2024 Jul;30(7):e14798. doi: 10.1111/cns.14798. CNS Neurosci Ther. 2024. PMID: 39015099 Free PMC article.
-
Quercetin alleviates depression in CUMS rats via modulating immune plasticity in the choroid plexus to inhibit hippocampal microglial activation.Eur J Pharmacol. 2025 Sep 15;1003:177860. doi: 10.1016/j.ejphar.2025.177860. Epub 2025 Jun 26. Eur J Pharmacol. 2025. PMID: 40581279
-
Neuroprotective effects of Rosavin via HIF-1α signaling in a rat model of ischemic stroke.Phytomedicine. 2025 Sep;145:157068. doi: 10.1016/j.phymed.2025.157068. Epub 2025 Jul 13. Phytomedicine. 2025. PMID: 40682944
-
Glucose Metabolic Reprogramming in Microglia: Implications for Neurodegenerative Diseases and Targeted Therapy.Mol Neurobiol. 2025 Jul;62(7):8204-8221. doi: 10.1007/s12035-025-04775-y. Epub 2025 Feb 22. Mol Neurobiol. 2025. PMID: 39987285 Review.
-
Intravenous versus inhalational maintenance of anaesthesia for postoperative cognitive outcomes in elderly people undergoing non-cardiac surgery.Cochrane Database Syst Rev. 2018 Aug 21;8(8):CD012317. doi: 10.1002/14651858.CD012317.pub2. Cochrane Database Syst Rev. 2018. PMID: 30129968 Free PMC article.
References
-
- Espinoza RT, Kellner CH (2022) Electroconvulsive Therapy[J]. N Engl J Med 386(7):667–672. 10.1056/NEJMra2034954 - PubMed
-
- Verwijk E, Comijs HC, Kok RM, Spaans HP, Stek ML, Scherder EJ (2012) Neurocognitive effects after brief pulse and ultrabrief pulse unilateral electroconvulsive therapy for major depression: a review[J]. J Affect Disord 140(3):233–243. 10.1016/j.jad.2012.02.024 - PubMed
-
- Bannerman DM, Sprengel R, Sanderson DJ, McHugh SB, Rawlins JN, Monyer H et al (2014) Hippocampal synaptic plasticity, Spatial memory and anxiety[J]. Nat Rev Neurosci 15(3):181–192. 10.1038/nrn3677 - PubMed
MeSH terms
Substances
Grants and funding
- 81,873,798/National Natural Science Foundation of China
- 81,873,798/National Natural Science Foundation of China
- 81,873,798/National Natural Science Foundation of China
- 81,873,798/National Natural Science Foundation of China
- 81,873,798/National Natural Science Foundation of China
- 81,873,798/National Natural Science Foundation of China
- 2007-2/Chongqing Medical Key Discipline Construction Project
- 2007-2/Chongqing Medical Key Discipline Construction Project
- 2007-2/Chongqing Medical Key Discipline Construction Project
- 2007-2/Chongqing Medical Key Discipline Construction Project
- 2007-2/Chongqing Medical Key Discipline Construction Project
- 2007-2/Chongqing Medical Key Discipline Construction Project
- cstc2019jcyj-msxmX0839/Chongqing Science and Technology Bureau under Grant
- cstc2019jcyj-msxmX0839/Chongqing Science and Technology Bureau under Grant
- cstc2019jcyj-msxmX0839/Chongqing Science and Technology Bureau under Grant
- cstc2019jcyj-msxmX0839/Chongqing Science and Technology Bureau under Grant
- cstc2019jcyj-msxmX0839/Chongqing Science and Technology Bureau under Grant
- cstc2019jcyj-msxmX0839/Chongqing Science and Technology Bureau under Grant
LinkOut - more resources
Full Text Sources
Medical

