Clinical Outcomes of Perioperative Immunotherapy in Resectable Non-Small Cell Lung Cancer
- PMID: 40587130
- PMCID: PMC12210081
- DOI: 10.1001/jamanetworkopen.2025.17953
Clinical Outcomes of Perioperative Immunotherapy in Resectable Non-Small Cell Lung Cancer
Abstract
Importance: Lung cancer remains a leading cause of cancer-related mortality, with early-stage non-small cell lung cancer (NSCLC) accounting for 40% to 45% of new diagnoses. Despite the adoption of perioperative chemoimmunotherapy, clinical data on its use and outcomes remain limited.
Objective: To evaluate clinical practice patterns and outcomes associated with neoadjuvant and adjuvant chemoimmunotherapy use in patients with resectable stage II to IIIA NSCLC.
Design, setting, and participants: This retrospective cohort study obtained data from the Flatiron Health database containing deidentified, patient-level, electronic health record-derived data from approximately 280 cancer clinics in the US. The cohort included adults diagnosed with stage II to IIIA NSCLC between January 1, 2020, and October 31, 2023, who underwent surgical resection. Included patients were receiving neoadjuvant chemoimmunotherapy (nivolumab or pembrolizumab with platinum-based doublet chemotherapy) or adjuvant chemoimmunotherapy (atezolizumab or pembrolizumab with or without chemotherapy) after the US Food and Drug Administration approval of these therapies.
Exposures: Neoadjuvant and adjuvant chemoimmunotherapy for resectable NSCLC.
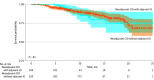
Main outcomes and measures: The primary outcome was clinical distant metastasis-free survival (DMFS), defined as time from treatment initiation to distant metastasis or death. Secondary outcomes included biomarker testing rates, time to treatment, and metastatic patterns.
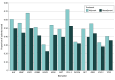
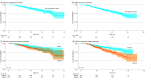
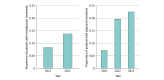
Results: Included in the analysis were 1334 patients with resectable stage II to IIIA NSCLC treated with immunotherapy, of whom 424 (median [IQR] age, 68 [63.0-74.0] years; 225 males [53.1%]) received chemoimmunotherapy with platinum-based doublet in the neoadjuvant setting and 910 (median [IQR] age, 69 [63.0-74.0] years; 441 males [48.5%]) received chemoimmunotherapy or immunotherapy alone in the adjuvant setting. The clinical DMFS at 18 months was 80.2% (95% CI, 75.0%-85.7%) for the neoadjuvant cohort and 83.0% (95% CI, 80.0%-86.0%) for the adjuvant cohort. Biomarker testing rates were higher in the adjuvant cohort compared to the neoadjuvant cohort (eg, programmed cell death ligand 1 testing: 72.2% vs 52.6%, P < .001). Common metastatic sites included brain, bone, and pleura, consistent across cohorts. Chemoimmunotherapy among patients who received surgery increased from 2022 to 2023 but remained under 30% of eligible patients (neoadjuvant setting: from 8.4% [82 of 966] to 13.8% [254 of 1842]; adjuvant setting: from 19.7% [372 of 1887] to 22.6% [401 of 1776]).
Conclusions and relevance: This retrospective cohort study found that chemoimmunotherapy was associated with favorable clinical DMFS outcomes in patients with resectable NSCLC. The findings highlight the need to address barriers to broader adoption of chemoimmunotherapy.
Conflict of interest statement
Figures
Comment in
- doi: 10.1001/jamanetworkopen.2025.17962
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

