Intermittent hypoxia aggravates asthma inflammation via NLRP3/IL-1β-dependent pyroptosis mediated by HIF-1α signalling pathway
- PMID: 40587235
- PMCID: PMC12273680
- DOI: 10.1097/CM9.0000000000003608
Intermittent hypoxia aggravates asthma inflammation via NLRP3/IL-1β-dependent pyroptosis mediated by HIF-1α signalling pathway
Abstract
Background: Asthma is a common chronic inflammatory airway disease and intermittent hypoxia is increasingly recognized as a factor that may impact disease progression. The present study investigated whether intermittent hypoxia (IH) could aggravate asthma by promoting hypoxia-inducible factor-1α (HIF-1α)/nucleotide-binding oligomerization domain (NOD)-like receptor pyrin domain-containing protein 3 (NLRP3)/interleukin (IL)-1β-dependent pyroptosis and the inflammatory response and further elucidated the underlying molecular mechanisms involved.
Methods: A total of 49 patients diagnosed with severe bronchial asthma and diagnosed by polysomnography were enrolled at Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology, between January 2022 and December 2022, and their general data and induced sputum were collected. BEAS-2B cells were treated with IL-13 and subjected to IH. An ovalbumin (OVA)-treated mouse model was also used to assess the effects of chronic intermittent hypoxia (CIH) on asthma. Pyroptosis, the inflammatory response, and related signalling pathways were assessed in vivo and in vitro .
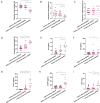
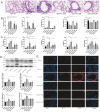
Results: In this study, as the apnoea and hypopnea index (AHI) increased, the proportion of patients with uncontrolled asthma increased. The proportions of neutrophils and the levels of IL-6, IL-8, HIF-1α and NLRP3 in induced sputum were related to the AHI. NLRP3-mediated pyroptosis, which could be mediated by the HIF-1α signalling pathway, was activated in IL-13 plus IH-treated BEAS-2B cells and in the lungs of OVA/CIH mice. HIF-1α downregulation significantly reduced lung pyroptosis and ameliorated neutrophil inflammation by modulating the NLRP3/IL-1β pathway both in vitro and in vivo . Similarly, pretreatment with LW6, an inhibitor of HIF-1α, effectively blocked the generation of inflammatory cytokines in neutrophils. In addition, administration of the NLRP3 activator nigericin obviously increased lung neutrophil inflammation.
Conclusions: Obstructive sleep apnoea-hypopnea syndrome (OSAHS) is a risk factor for asthma exacerbation. IH aggravates neutrophil inflammation in asthma via NLRP3/IL-1β-dependent pyroptosis mediated by the HIF-1α signalling pathway, which should be considered a potential therapeutic target for the treatment of asthma with OSAHS.
Keywords: Asthma; Hypoxia-inducible factor-1α (HIF-1α); Inflammation; Interleukin-1β; Intermittent hypoxia; NOD-like receptor pyrin domain-containing protein 3 (NLRP3).
Copyright © 2025 The Chinese Medical Association, produced by Wolters Kluwer, Inc. under the CC-BY-NC-ND license.
Conflict of interest statement
None.
Figures





References
-
- Zhang H Zhou L Zhou Y Wang L Jiang W Liu L, et al. Intermittent hypoxia aggravates non-alcoholic fatty liver disease via RIPK3-dependent necroptosis-modulated Nrf2/NFκB signaling pathway. Life Sci 2021;285:119963. doi: 10.1016/j.lfs.2021.119963. - PubMed
-
- Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: A review. JAMA 2020;323:1389–1400. doi: 10.1001/jama.2020.3514. - PubMed
-
- Strausz S Ruotsalainen S Ollila HM Karjalainen J Kiiskinen T Reeve M, et al. Genetic analysis of obstructive sleep apnoea discovers a strong association with cardiometabolic health. Eur Respir J 2021;57:2003091. doi: 10.1183/13993003.03091-2020. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

