In utero exposure to electronic cigarette carriers alters craniofacial morphology
- PMID: 40587499
- PMCID: PMC12208421
- DOI: 10.1371/journal.pone.0327190
In utero exposure to electronic cigarette carriers alters craniofacial morphology
Abstract
Objectives: Despite the popularity of electronic nicotine delivery systems (ENDS), there is currently a lack of regulation and consistency regarding the formulation of the e-liquids that undergo combustion in use. The two main constituents of most e-liquids are the humectants propylene glycol (PG) and glycerol (vegetable glycerin, VG). E-liquids consist of a ratio of these two components with PG utilized to increase the "throat hit" effect and VG used to increase visible vapor. As PG-based e-liquids are known to generate more carcinogenic carbonyls and increase the uptake of nicotine, many commercial products have moved toward a more VG-centric formulation to reduce potential harm. The purpose of this study was to test the hypothesis that a common VG-based formulation (30/70 PG/VG) would result in fewer negative effects on craniofacial growth compared to an evenly concentrated formulation (50/50 PG/VG) in the absence of nicotine.
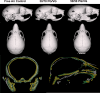
Materials and methods: Adult breeder mice were utilized to generate in utero ENDS component exposed litters including free air exposure (control), 30/70 PG/VG, and 50/50 PG/VG groups. The resulting pups were assessed at postnatal day 14 for skull morphology.
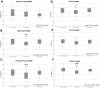
Results: Data demonstrate significant reductions in body weight, facial, and cranial dimensions, where there was a significant reduction in growth for the 30/70 PG/VG exposed group. There were no significant differences found between control and 50/50 PG/VG.
Conclusions: These results suggest the overall movement to a more VG-centric ENDS formulation may not result in reduced profile for health concerns. Further, it suggests that PG/VG are not a harmless carrier and now popular nicotine-free ENDS formulation may not be considered safe for use in pregnant populations.
Copyright: © 2025 Richlak et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

