Evidence for alcohol-mediated hemolysis and erythrophagocytosis
- PMID: 40587925
- PMCID: PMC12268204
- DOI: 10.1016/j.redox.2025.103742
Evidence for alcohol-mediated hemolysis and erythrophagocytosis
Abstract
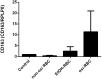
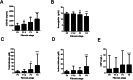
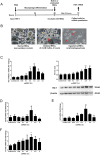
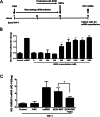
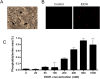
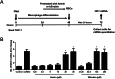
Alcohol-related liver disease (ALD) is the most common liver disease worldwide; however, its underlying molecular mechanisms remain poorly understood. Here, we identify ethanol-mediated hemolysis and erythrophagocytosis as major contributors to ALD pathogenesis using both in vitro and in vivo models, as well as surrogate markers such as heme oxygenase-1 (HO-1) and CD163, a scavenger receptor for hemoglobin-haptoglobin complexes. A key initial observation was the direct optical evidence of serum hemolysis in heavy drinkers, which diminished after one week of alcohol withdrawal. In parallel, soluble CD163 (sCD163) levels declined during alcohol detoxification correlating with liver damage and fibrosis stages. Moreover, red blood cells (RBCs) from heavy drinkers exhibited increased fragility under hemolytic stress. In ethanol-fed mice, we also observed serum hemolysis. Erythrophagocytosis in liver tissue was visualized by co-localization of CD163 and hemoglobin autofluorescence. In vitro studies confirmed that ethanol - at concentrations transiently present in the upper gastrointestinal tract during alcohol ingestion - directly induces hemolysis and primes RBCs for erythrophagocytosis through eryptosis, marked by externalization of phosphatidylserine. Both heme, released during hemolysis, and bilirubin, its degradation product, further amplified erythrophagocytosis at clinically relevant concentrations, suggesting a self-perpetuating cycle. The antioxidant N-acetylcysteine efficiently blocked ethanol-induced RBC priming for erythrophagocytosis. In conclusion, alcohol triggers a cascade of hemolysis, eryptosis, and erythrophagocytosis that may contribute to the pathogenesis of alcoholic hepatitis and end-stage ALD. sCD163 could serve as a noninvasive marker of hemolysis-associated macrophage activation. This mechanism opens new avenues for antioxidant-based therapies and may help to explain typical iron abnormalities, including ferroptosis, and hyperbilirubinemia in ALD.
Keywords: Alcohol-related liver disease; Alcoholic hepatitis; Bilirubin; CD163; Eryptosis; Erythrophagocytosis; Ferroptosis; Heme; Hemoglobin; Hemolysis; Iron overload; Liver cirrhosis; Red blood cell.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- World Health Organization Global status report on alcohol and health. 2018. http://www.who.int/substance_abuse/publications/global_alcohol_report/en...
-
- Mueller S., Heilig M. Springer International Publishing; 2023. Alcohol and alcohol-related Diseases.
-
- Mueller S., Rausch V. The role of iron in alcohol-mediated hepatocarcinogenesis. Adv. Exp. Med. Biol. 2015;815:89–112. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

