Joint spatial associations of amyloid beta and tau pathology in Down syndrome and preclinical Alzheimer's disease: Cross-sectional associations with early cognitive impairments
- PMID: 40588723
- PMCID: PMC12208798
- DOI: 10.1002/alz.70424
Joint spatial associations of amyloid beta and tau pathology in Down syndrome and preclinical Alzheimer's disease: Cross-sectional associations with early cognitive impairments
Abstract
Introduction: Individuals with Down syndrome (DS) have elevated risks for Alzheimer's disease (AD) due to amyloid beta (Aβ) precursor protein overexpression, with nearly all developing AD pathology by age 40 at autopsy. This study examined spatial associations between Aβ and tau burden in DS and neurotypical aging.
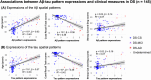
Methods: Data included 145 DS (25-67 years) and 191 neurotypical aging individuals (63-89 years). Regional Aβ and tau positron emission tomography outcomes were analyzed using multiset canonical correlation analysis to identify joint Aβ/tau spatial patterns, with regression models assessing associations with age and cognition.
Results: For a given Aβ burden, cognitively stable DS individuals exhibited relatively higher tau burden than neurotypical aging, while DS mild cognitive impairment/AD individuals exhibited more widespread pathology. Joint Aβ/tau patterns were associated with episodic memory impairment in DS and, as the disease progresses, executive dysfunction.
Discussion: DS exhibits overlapping and distinct AD-related neuropathology features, emphasizing the importance of biomarkers for early detection and intervention.
Highlights: There are distinct amyloid beta (Aβ) and tau spatial patterns in Down syndrome (DS): For a given level of Aβ burden, individuals with DS exhibited greater and more widespread tau burden compared to neurotypical aging, even before a clinical diagnosis of dementia. Aβ-associated tau burden was linked to episodic memory impairment in DS prior to dementia, with executive dysfunction emerging as the disease progressed, highlighting the sequential impact of pathology on cognition. The unique pattern of early striatal Aβ accumulation in DS supports its use as a potential biomarker for tracking disease progression and guiding clinical trial inclusion criteria for Alzheimer's disease interventions in DS.
Keywords: Alzheimer's disease; Down syndrome; amyloid; memory; multivariate analysis; preclinical; tau.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no conflicts of interest. Author disclosures are available in the supporting information.
Figures

Similar articles
-
Prediction of amyloid and tau brain deposition and cognitive decline in people with Down syndrome using plasma biomarkers: a longitudinal cohort study.Lancet Neurol. 2025 Jul;24(7):591-600. doi: 10.1016/S1474-4422(25)00158-9. Lancet Neurol. 2025. PMID: 40541209 Free PMC article.
-
Timeline to symptomatic Alzheimer's disease in people with Down syndrome as assessed by amyloid-PET and tau-PET: a longitudinal cohort study.Lancet Neurol. 2024 Dec;23(12):1214-1224. doi: 10.1016/S1474-4422(24)00426-5. Lancet Neurol. 2024. PMID: 39577922
-
Longitudinal study of body mass index in relation to Alzheimer's disease pathology and symptomatology in Down syndrome.Alzheimers Dement. 2025 Jun;21(6):e70387. doi: 10.1002/alz.70387. Alzheimers Dement. 2025. PMID: 40545561 Free PMC article.
-
CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2017 Mar 22;3(3):CD010803. doi: 10.1002/14651858.CD010803.pub2. Cochrane Database Syst Rev. 2017. PMID: 28328043 Free PMC article.
-
18F PET with flutemetamol for the early diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2017 Nov 22;11(11):CD012884. doi: 10.1002/14651858.CD012884. Cochrane Database Syst Rev. 2017. PMID: 29164602 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

