Baicalin confers neuroprotection in animal models of stroke through its antioxidant and anti-apoptotic effects
- PMID: 40589105
- PMCID: PMC12329058
- DOI: 10.4142/jvs.25055
Baicalin confers neuroprotection in animal models of stroke through its antioxidant and anti-apoptotic effects
Abstract
Importance: Ischemic stroke leads to neuronal cell death due to a lack of oxygen and glucose. Baicalin is a flavonoid that has antioxidant and anti-inflammatory properties.
Objective: The aim of this study is to elucidate the anti-oxidant and anti-apoptotic effects of baicalin in animal models of stroke.
Methods: Vehicle or baicalin (100 mg/kg) was administered intraperitoneally immediately after the middle cerebral artery occlusion (MCAO) surgery. Neurobehavioral tests were conducted 24 h post-MCAO and brain tissue was isolated to assess histopathological changes and apoptosis-associated protein expression. Additionally, reactive oxygen species (ROS) and lipid peroxidation (LPO) assays were performed to evaluate oxidative stress.
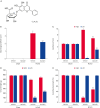
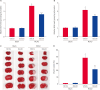
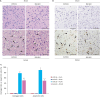
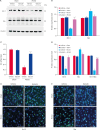
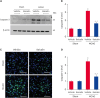
Results: MCAO animals exhibited severe neurological deficits, which were significantly alleviated by baicalin treatment. Baicalin mitigated the up-regulation in ROS and LPO levels induced by surgery. MCAO damage led to severe histopathological lesions and an increase in terminal deoxynucleotidyl transferase dUTP nick end labeling-positive reactions, these alterations were alleviated by baicalin treatment. MCAO damage decreases the expression of Bcl-2 and increases the expression of Bax, baicalin alleviates these changes. Baicalin also attenuated the upregulation of caspase-3 expression caused by MCAO injury.
Conclusions and relevance: These results can suggest evidence that baicalin exerts neuroprotective effects by preventing apoptosis during cerebral ischemia. In conclusion, baicalin acts as a potent neuroprotective agent through its antioxidant and anti-apoptotic effects on neuronal cell damage.
Keywords: Baicalin; cerebral ischemia; neuroprotection; stroke.
© 2025 The Korean Society of Veterinary Science.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Chlorogenic acid alleviates neurobehavioral disorders and brain damage in focal ischemia animal models.Neurosci Lett. 2021 Aug 24;760:136085. doi: 10.1016/j.neulet.2021.136085. Epub 2021 Jun 24. Neurosci Lett. 2021. PMID: 34174343
-
Neuroprotective effects of Rosavin via HIF-1α signaling in a rat model of ischemic stroke.Phytomedicine. 2025 Sep;145:157068. doi: 10.1016/j.phymed.2025.157068. Epub 2025 Jul 13. Phytomedicine. 2025. PMID: 40682944
-
Remote Ischemic Postconditioning Improve Cerebral Ischemia-Reperfusion Injury Induced Cognitive Dysfunction through Suppressing Mitochondrial Apoptosis in Hippocampus via TK/BK/B2R-Mediated PI3K/AKT.Mol Neurobiol. 2025 Aug;62(8):10652-10669. doi: 10.1007/s12035-025-04864-y. Epub 2025 Apr 14. Mol Neurobiol. 2025. PMID: 40229456 Free PMC article.
-
The Effects of IRL-1620 in Post-ischemic Brain Injury: A Systematic Review and Meta-analysis of Experimental Studies.Neurocrit Care. 2024 Oct;41(2):665-680. doi: 10.1007/s12028-024-01994-4. Epub 2024 May 9. Neurocrit Care. 2024. PMID: 38724864
-
Cooling for cerebral protection during brain surgery.Cochrane Database Syst Rev. 2015 Jan 28;1(1):CD006638. doi: 10.1002/14651858.CD006638.pub3. Cochrane Database Syst Rev. 2015. PMID: 25626888 Free PMC article.
References
-
- Ingall T. Stroke--incidence, mortality, morbidity and risk. J Insur Med. 2004;36(2):143–152. - PubMed
-
- Hankey GJ. Stroke. Lancet. 2017;389(10069):641–654. - PubMed
-
- Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018;38(2):208–211. - PubMed
-
- Hou ST, MacManus JP. Molecular mechanisms of cerebral ischemia-induced neuronal death. Int Rev Cytol. 2002;221:93–148. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

