Integration of magnetic graphene oxide and office waste paper-derived fibrillated cellulose into a composite adsorbent for effective tetracycline elimination
- PMID: 40589466
- PMCID: PMC12207975
- DOI: 10.1039/d5ra00877h
Integration of magnetic graphene oxide and office waste paper-derived fibrillated cellulose into a composite adsorbent for effective tetracycline elimination
Abstract
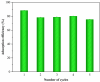
Utilizing office waste paper for environmental treatments has garnered significant interest from the research community, as it addresses both waste management and the development of low-cost sustainable adsorbents. In this study, a novel approach was developed by integrating office waste paper-derived fibrillated cellulose (PFC) with magnetic graphene oxide (M-GO) to develop a composite adsorbent for the removal of tetracycline (TC) from aqueous solutions. PFC was isolated via an alkali-acid treatment process; graphene oxide (GO) was synthesized using a modified Hummers' method and subsequently functionalized with magnetite nanoparticles to produce M-GO. The M-GO/PFC composite was then prepared using an ultrasound-assisted mixing technique, followed by lyophilization. Materials were characterized using Fourier-transform infrared (FTIR) spectroscopy, X-ray powder diffraction (XRD), Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), field-emission scanning electron microscopy (FE-SEM), energy-dispersive X-ray (EDX) spectroscopy, N2 adsorption-desorption isotherms, and vibrating sample magnetometry (VSM). The effects of solution pH, adsorbent dose, and ionic strength on TC removal by the composite adsorbent were systematically investigated. Adsorption kinetics was analyzed using the pseudo-first-order, pseudo-second-order, and Elovich models, suggested to fit well with the pseudo-second-order kinetic model with an initial rate of 18.09 mg g-1 min-1. Adsorption isotherms were evaluated using the Langmuir, Freundlich, Sips, and Temkin models, of which the Sips model best described the experimental data, yielding a maximum adsorption capacity of 130.11 mg g-1. Recyclability testing was carried out through five successive adsorption-desorption cycles, indicating a stable adsorption performance of the composite adsorbent with 12.9% decrease between the first and the fifth cycle. These findings suggest that the M-GO/PFC composite is a promising and effective adsorbent for the removal of TC and potentially other water-soluble antibiotics from aqueous environments.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures













References
-
- Kong Y. Zhuang Y. Han K. Shi B. Colloids Surf., A. 2020;588:124360.
-
- Zhao Y. Yang Q. E. Zhou X. Wang F.-H. Muurinen J. Virta M. P. Brandt K. K. Zhu Y.-G. Crit. Rev. Environ. Sci. Technol. 2021;51:2159–2196.
-
- Xu L. Zhang H. Xiong P. Zhu Q. Liao C. Jiang G. Sci. Total Environ. 2021;753:141975. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

