This is a preprint.
Selective inhibition of MR1-restricted T cell activation by a novel MR1-targeting nanobody
- PMID: 40589544
- PMCID: PMC12208488
- DOI: 10.1101/2025.06.11.659204
Selective inhibition of MR1-restricted T cell activation by a novel MR1-targeting nanobody
Abstract
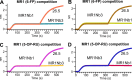
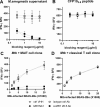
MR1 is a non-polymorphic, ubiquitously expressed, MHC class I-like antigen-presenting molecule that presents small-molecule metabolites to T cells. Studies have shown that MR1 plays a role in microbial infection, inflammation, and tumor immunity. The antigens it presents include metabolites of microbial and self-origin as well as small-molecule drugs and form stable complexes with MR1 that are displayed on the cell surface to activate T cells. However, unlike classical MHC I and II molecules, the fundamental biology of MR1 remains poorly understood, particularly the mechanisms governing antigen loading and intracellular trafficking. This knowledge gap is largely due to the lack of molecular tools available to precisely manipulate MR1 function. In this study, we describe a high-affinity (1.6 nM K D ) anti-MR1 nanobody, MR1Nb1. We characterize the binding of this nanobody including affinity by ELISA and kinetics by BLI. Crucially, we map the binding epitope of MR1Nb1 on MR1 by HDX-MS, providing key insights into the mechanism through which it blocks MR1T cell activation. In functional assays MR1Nb1 effectively and specifically blocks MAIT cell activation by cells infected with M. tuberculosis or treated with M. smegmatis supernatant. This nanobody represents a unique and versatile tool for the field, as it can be produced inexpensively and expressed intracellularly within antigen-presenting cells. Hence, our study provides a powerful new molecular probe for dissecting the mechanistic underpinnings of MR1 biology and uncover its broader roles in immunity.
Keywords: MAIT; MR1; Nanobody; T cell; tuberculosis.
Conflict of interest statement
Conflicts of Interest J.E.B. reports personal fees from Scorpion Therapeutics, Reactive therapeutics and Olema Oncology, and research grants from Novartis. T.A.B. and F.G.T. are co-founders of AlpaCure LLC. All other authors declare no competing interests.
Figures



References
-
- Corbett AJ et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014. May;509(7500):361–5. - PubMed
-
- Kjer-Nielsen L et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012. Nov 29;491(7426):717–23. - PubMed
-
- Hashimoto K et al. A Gene Outside the Human MHC Related to Classical HLA Class I Genes. Science. 1995. Aug 4;269(5224):693–5. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
