Measuring neurological severity and complexity in acute setting: the modified Neurological Impairment Scale
- PMID: 40589564
- PMCID: PMC12207106
- DOI: 10.1136/bmjno-2025-001107
Measuring neurological severity and complexity in acute setting: the modified Neurological Impairment Scale
Abstract
Background: Given the increasing diversity among neurological patients, standardised protocols are essential for evaluating the severity and complexity of the variety of conditions. The aim of the present work was to standardise the assessment of the severity and complexity of neurological impairment in an acute setting by using a modified version of the Neurological Impairment Scale (mNIS).
Methods: Consecutively hospitalised neurological inpatients underwent a multidimensional standardised assessment of multimorbidity, frailty, functional dependency and neurological impairment using mNIS and other validated scales. Inter-rater reliability of the mNIS total and subscores was evaluated. Construct validity was assessed separately in patients with cerebrovascular disease, performing correlations between corresponding subscores of mNIS, original NIS and National Institutes of Health Stroke Scale. mNIS Complexity Index (mNIS-CI) for neurological severity was used to classify patients into subtle, mild, moderate and severe impairment.
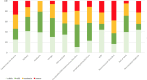
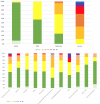
Results: 1081 neurological patients admitted to a neurological ward from the emergency setting were enrolled. The inter-rater reliability was remarkable for mNIS total and subscores (intraclass correlation coefficient 0.90, 95% CI 0.82 to 0.95). The mNIS showed strong construct validity for total and subscores compared with other clinical scales (r 0.47-0.97, p<0.001) and 52.7% of patients scored at least one in one of the four newly listed items. The stratification of patients according to mNIS-CI exhibited high construct validity, distinguishing the extent of impairment and involved domains.
Conclusions: The mNIS is valuable for measuring neurological severity and complexity in acute inpatients and holds significant potential for application in different settings.
Keywords: CEREBROVASCULAR DISEASE; CLINICAL NEUROLOGY.
Copyright © Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
APa received personal compensation as a consultant/scientific advisory board member for Biogen, Lundbeck, Roche, Nutricia, General Healthcare (GE). APa has been supported by grants of the Italian Ministry of University and Research PRIN COCOON (2017MYJ5TH) and PRIN 2021 RePlast (20202THZAW), the H2020 IMI IDEA-FAST (ID853981), Italian Ministry of Health, Grant/Award Number: RF-2018-12366209, RF-2019-12369272 and PNRR-Health PNRR-MAD-2022-12376110. IM, TC, NZ, CZ, MC, EG, SG, BSNWG, AM, CA report no competing interests. LT-S is director of the UK Rehabilitation Outcomes Collaborative (UKROC) and was the lead developer of the UKROC tools including the Neurological Impairment Scale. However, neither she nor her employing institution has any financial interest in the tools which are disseminated free of charge. APi received consultancy/speaker fees from AbbVie, Angelini, Bial, Lundbeck, Roche and Zambon pharmaceuticals and the Movement Disorder Society. APi has been supported by grants of Airalzh Foundation AGYR2021 Life-Bio Grant, The LIMPE-DISMOV Foundation Segala Grant 2021, the Italian Ministry of University and Research PRIN COCOON (2017MYJ5TH) and PRIN 2021 RePlast (20202THZAW), the H2020 IMI IDEA-FAST (ID853981), Italian Ministry of Health, Grant/Award Number: RF-2018-12366209 and PNRR-Health PNRR-MAD-2022-12376110.
Figures
References
LinkOut - more resources
Full Text Sources
