From hormones to neurodegeneration: how FSH drives Alzheimer's disease
- PMID: 40589620
- PMCID: PMC12206753
- DOI: 10.3389/fnagi.2025.1578439
From hormones to neurodegeneration: how FSH drives Alzheimer's disease
Abstract
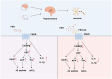
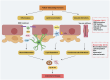
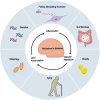
The role and function of follicle-stimulating hormone in the gonads have been extremely studied. However, recent research has begun to explore the relationship between elevated follicle-stimulating hormone levels and the prevalence of extragonadal disorders, particularly in perimenopausal and postmenopausal women. These disorders include endometrial cancer, osteoporosis, obesity, and atherosclerosis. This review provides new insights into the relationship between follicle-stimulating hormone and the development of age-related diseases, with a focus on Alzheimer's disease. Follicle-stimulating hormone does not act alone in promoting Alzheimer's disease but often works in conjunction with inflammation, lipid accumulation, and vascular alterations. Furthermore, follicle-stimulating hormone synergizes with obesity, gut microbiota, autophagy, and aging, creating conditions that facilitate the onset and progression of Alzheimer's disease. This review also summarizes the therapeutic potential of FSH-blocking antibodies in treating these diseases.
Keywords: Alzheimer’s disease; FSH-blocking antibodies; aging; follicle-stimulating hormone; lipid accumulation; neuroinflammation.
Copyright © 2025 Xue, Zuo, Wang and Qi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Apatiga-Perez R., Soto-Rojas L. O., Campa-Cordoba B. B., Luna-Viramontes N. I., Cuevas E., Villanueva-Fierro I., et al. (2022). Neurovascular dysfunction and vascular amyloid accumulation as early events in Alzheimer's disease. Metab. Brain Dis. 37, 39–50. doi: 10.1007/s11011-021-00814-4, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

