Sex-specific cytokine signatures as predictors of anti-PD1 therapy response in non-small cell lung cancer
- PMID: 40589738
- PMCID: PMC12206799
- DOI: 10.3389/fimmu.2025.1583421
Sex-specific cytokine signatures as predictors of anti-PD1 therapy response in non-small cell lung cancer
Abstract
Background: The introduction of immune checkpoint inhibitors (ICI) as first-line therapy in the treatment of non-small cell lung cancer has dramatically improved response rates. However, more than half of NSCLC patients receiving ICI fail to have a durable response to treatment and therefore the identification of circulating biomarkers to improve patient stratification is required. Cytokines and chemokines are critical mediators of immune responses, affecting tumor progression and immune evasion mechanisms. Thus, profiling circulating cytokines is particularly important, as these signaling molecules may provide valuable insights into predicting response and resistance to ICI.
Methods: Twenty-four circulating chemokines and cytokines were profiled in NSCLC patient plasma collected either prior to treatment or while on-treatment with anti-PD1 therapy and correlated to treatment response as well as to progression-free survival (PFS) and overall survival (OS). Sex-disparities in correlations of cytokines to response and survival was analyzed.
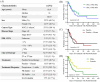
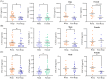
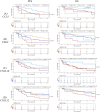
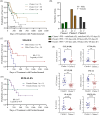
Results: Regardless of sex, baseline levels of CCL5/RANTES were associated with anti-PD1 treatment response, while CXCL5 was associated with response in males and CXCL10 was elevated in female responders to anti-PD1 treatment. VEGF and CD40L were associated with short PFS and OS, while CCL5 and CXCL5 were correlated to longer PFS and OS. Sex disparities in baseline cytokine levels were also observed. CCL5 was significantly correlated to PFS and OS in females but not males, and CXCL10 was found to be predictive of longer OS in females only. VEGF was found to be a better predictor of response t to anti-PD1 in females, while CXCL12 was found to be associated with short PFS and OS in males but not females. Uniform Manifold Approximation and Projection (UMAP) dimension reduction method and k-means clustering analysis identified a cluster of male patients with short PFS characterized by elevated baseline levels of VEGF, CCL4, CCL5, CCL20, and CXCL2.
Conclusions: Plasma cytokine levels can be useful biomarkers for predicting response to anti-PD1 therapy in NSCLC patients. However, the data presented in this study demonstrate that sex needs to be considered as an important variable in biomarker studies in immuno-oncology due to sex disparities in correlations of cytokines to anti-PD1 treatment response.
Keywords: CXCL10; CXCL12; NSCLC; chemokine; cytokine; immune checkpoint inhibitor; sex disparity.
Copyright © 2025 Taylor, Cheema, Asleh, Finn, Abdelsalam and Ouellette.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

