Utility of diaphragm dome height as a marker of operational lung volume changes, disease burden and exacerbations in patients with mild-to-moderate COPD: an observational study within the CanCOLD cohort
- PMID: 40589904
- PMCID: PMC12208606
- DOI: 10.1183/23120541.01042-2024
Utility of diaphragm dome height as a marker of operational lung volume changes, disease burden and exacerbations in patients with mild-to-moderate COPD: an observational study within the CanCOLD cohort
Abstract
Background: Dynamic hyperinflation is central to dyspnoea, exercise limitation and exacerbations in COPD. While studied previously in moderate-to-severe COPD, the relevance of diaphragm dome height (DDH) on clinically important outcomes has been under-investigated in mild-to-moderate COPD.
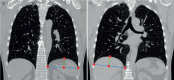
Methods: Canadian Cohort Obstructive Lung Disease (CanCOLD) participants with spirometry-confirmed COPD, symptom-limited incremental cardiopulmonary exercise testing and computed tomography image data were included. Base-to-apex left DDH (LDDH) and right DDH (RDDH) were automatically segmented, with increased height implying less flattening and thus less hyperinflation. Dynamic hyperinflation was defined as ≥150 mL reduction in inspiratory capacity (IC) from rest to peak exercise. Cross-sectional linear regression models were fitted between LDDH and RDDH (predictor variables) with peak IC (ICpeak), peak workload (W peak), forced expiratory volume in 1 s (FEV1) and COPD Assessment Test (CAT) score (outcome variables), and in longitudinal (Anderson-Gill) models with "symptom-based" and "event-based" exacerbations. Results are reported as parameter estimates or hazard ratios (HRs) with 95% confidence intervals per interquartile range dome height increment.
Results: Amongst 304 participants (mean±sd age 64.7±10.3 years, 41.8% female, 44.4% with mild COPD), each LDDH and RDDH increment, respectively, was associated with ICpeak (0.21 (95% CI 0.13-0.29) L and 0.13 (95% CI 0.07-0.19) L), W peak (9.54 (95% CI 5.03-14.04) W and 6.04 (95% CI 2.45-9.62) W), FEV1 (0.17 (95% CI 0.10-0.25) L and 0.08 (95% CI 0.02-0.14) L) and CAT score (-1.36 (95% CI -2.39- -0.33) and -0.82 (95% CI -1.63-0.00)). LDDH alone was associated with both symptom-based (HR 0.82 (95% CI 0.74-0.91)) and event-based (HR 0.83 (95% CI 0.73-0.95)) exacerbations. Of 167 out of 304 participants with confirmed dynamic hyperinflation (ΔIC -0.47±0.25 L), LDDH alone was associated with all outcomes (ICpeak, W peak, FEV1, CAT and symptom-based/event-based exacerbations).
Conclusions: LDDH appears to be a clinically important marker for operational lung volume changes, lung function, exercise performance, disease burden and exacerbations in mild-to-moderate COPD.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: S.A. Beydoun reports support for the present manuscript from the Respiratory Epidemiology and Clinical Research Unit at McGill University Health Centre (MUHC). D. Genkin reports support for the present manuscript from NSERC-PGS-D grant. P.Z. Li reports constancy fees from VIDA Diagnostics. F. Maltais reports grants from GlaxoSmithKline, AstraZeneca, Sanofi, Novartis and Grifols; consultancy fees from AstraZeneca; payment or honoraria for lectures, presentations, manuscript writing or educational events from GlaxoSmithKline and AstraZeneca; and stock or stock options with Oxynov. J. Bourbeau reports grants from the Canadian Institutes of Health Research, FRQS Respiratory Health Network, MUHC Foundation, AstraZeneca, GlaxoSmithKline, Novartis and Trudel; and payment or honoraria for lectures, presentations, manuscript writing or educational events from CHEST, Canadian Thoracic Society, L'Association des Pneumologues de la Province de Québec, AstraZeneca, GlaxoSmithKline, Inogen and Roche. W. Tan reports participation on a data safety monitoring board or advisory board with Sanofi. D.D. Sin reports grants from Nextone; payment or honoraria for lectures, presentations, manuscript writing or educational events from GlaxoSmithKline, AstraZeneca and Boehringer Ingelheim; and participation on a data safety monitoring board or advisory board with the National Heart, Lung, and Blood Institute and is Deputy Chief Editor of the European Respiratory Journal. S.D. Aaron reports consultancy fees from GlaxoSmithKline, AstraZeneca, Sanofi and Methapharm; and payment or honoraria for lectures, presentations, manuscript writing or educational events from GlaxoSmithKline, AstraZeneca and Sanofi. K.R. Chapman reports grants from BMS, Bellus, AstraZeneca, GlaxoSmithKline, Sanofi, Regeneron, Takeda and Novartis; consultancy fees from AstraZeneca, GlaxoSmithKline, Inhibrix, Mereo, Regeneron, Sanofi and Takeda; payment or honoraria for lectures, presentations, manuscript writing or educational events from Valeo, Sanofi, Novartis, GlaxoSmithKline and Takeda; participation on a data safety monitoring board with Intellia (ITL-3001-CL-101 DSMB); and a leadership role with AlphaNet Canada. P. Hernandez reports support for the present study from the Canadian Institutes of Health Research; grants from Grifols, Cyclomedica, Boehringer Ingelheim and Wave Life Sciences; payment or honoraria for lectures, presentations, manuscript writing or educational events from the Canadian Thoracic Society, GlaxoSmithKline and AstraZeneca; participation on a data safety monitoring board or advisory board with AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Merck and Takeda; and a leadership role with the Canadian Thoracic Society. D.D. Marciniuk reports grants from AstraZeneca, Boehringer Ingelheim, Canadian Institutes of Health Research, GlaxoSmithKline, Grifols, Lung Association of Saskatchewan, Lung Health Institute of Canada, Novartis, Sanofi, Saskatchewan Health Research Foundation and Schering-Plough; support for attending meetings from the University of Saskatchewan; a leadership role with the Saskatchewan Health Research Foundation; and is an employee of the University of Saskatchewan and Deputy Editor of CHEST. B.L. Walker reports payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, GlaxoSmithKline and Sanofi. M. Kirby reports consultancy fees from VIDA Diagnostics. B.A. Ross reports support for the present study from the McGill University Health Centre (MUHC) Department of Medicine Contract Academic Staff (CAS) Research Award, and a Respiratory Epidemiology and Clinical Research Unit Summer Studentship Research Award; grants from the Quebec Respiratory Health Network, Ministère de l’Éducation et le Ministère de l'Enseignement Supérieur Innovation and Office of Innovation and Partnerships (I+P) of McGill University (McGill University and Thorasys Inc.), McGill Interdisciplinary Initiative in Infection and Immunity (MI4) Pfizer Early Career Investigator Award (ECA), MUHC Foundation/MCI Respiratory Research Campaign Innovation Grant, MUHC Department of Medicine CAS Research Award, MGH Foundation Research Award, Trudell Medical International Unrestricted Investigator-Initiated Operating Grant, AstraZeneca Unrestricted Investigator-Initiated Operating Grant; payment or honoraria for lectures, presentations, manuscript writing or educational events from GlaxoSmithKline, AstraZeneca, COVIS, Canadian Thoracic Society, McGill University CPD, L'Association des Pneumologues de la Province de Québec, CHEST, Alberta Kinesiology Association and Respiplus; and receipt of equipment, materials, drugs, medical writing, gifts or other services from Amazentis, Thorasys Inc. and Restech. The remaining authors have nothing to disclose.
Figures

Similar articles
-
Inhaled corticosteroids with combination inhaled long-acting beta2-agonists and long-acting muscarinic antagonists for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2023 Dec 6;12(12):CD011600. doi: 10.1002/14651858.CD011600.pub3. Cochrane Database Syst Rev. 2023. PMID: 38054551 Free PMC article.
-
Immunostimulants versus placebo for preventing exacerbations in adults with chronic bronchitis or chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2022 Nov 14;11(11):CD013343. doi: 10.1002/14651858.CD013343.pub2. Cochrane Database Syst Rev. 2022. PMID: 36373977 Free PMC article.
-
Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2012 Sep 12;2012(9):CD006829. doi: 10.1002/14651858.CD006829.pub2. Cochrane Database Syst Rev. 2012. PMID: 22972099 Free PMC article.
-
Inhaled corticosteroids versus placebo for stable chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2023 Mar 27;3(3):CD002991. doi: 10.1002/14651858.CD002991.pub4. Cochrane Database Syst Rev. 2023. PMID: 36971693 Free PMC article.
-
Telehealth interventions: remote monitoring and consultations for people with chronic obstructive pulmonary disease (COPD).Cochrane Database Syst Rev. 2021 Jul 20;7(7):CD013196. doi: 10.1002/14651858.CD013196.pub2. Cochrane Database Syst Rev. 2021. PMID: 34693988 Free PMC article.
References
-
- Leung C, Bourbeau J, Sin DD, et al. The prevalence of chronic obstructive pulmonary disease (COPD) and the heterogeneity of risk factors in the Canadian population: results from the Canadian Obstructive Lung Disease (COLD) Study. Int J Chron Obstruct Pulmon Dis 2021; 16: 305–320. doi: 10.2147/COPD.S285338 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
