Treatment choice for adult patients with moderate-to-severe asthma: the TAILOR study
- PMID: 40589911
- PMCID: PMC12208605
- DOI: 10.1183/23120541.00869-2024
Treatment choice for adult patients with moderate-to-severe asthma: the TAILOR study
Abstract
Background: In patients with uncontrolled asthma treated with medium dose inhaled corticosteroid/long-acting β2-agonist (ICS/LABA), the Global Initiative for Asthma (GINA) recommends to increase to high-dose ICS/LABA or to start therapy consisting of medium-dose ICS/LABA+LAMA (long-acting muscarinic antagonist). Adding LAMA on top of high-dose ICS/LABA is not recommended for Step 4 asthma patients, yet it is used in the real world. Patient characteristics influencing treatment step-up are unknown. The objectives of the present study were to identify determinants of step-up option (high-dose ICS/LABA, medium-dose ICS/LABA+LAMA, high-dose ICS/LABA+LAMA) in patients with moderate to severe asthma.
Methods: A retrospective cohort study using three primary care databases (IPCI, HSD, CPRD GOLD) and one prescription database (Aarhus) included asthma patients with step-up after ≥3 months use of medium-dose ICS/LABA, from January 2010 to April 2020. Characteristics of patients were described, and determinants of choice for medium-dose ICS/LABA+LAMA or high dose ICS/LABA were investigated.
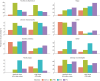
Results: 492 639 adults with asthma and ≥1 year of database history were identified and 25 558 were eligible for analysis. 6126 patients stepped-up to medium-dose ICS/LABA+LAMA and 18 947 patients stepped-up to high-dose ICS/LABA. Determinants for step-up to medium-dose ICS/LABA+LAMA were higher age and presence of COPD whereas history of atopy lowered this choice. Other covariates were differentially associated with specific treatment step-up depending on the databases.
Conclusion: In uncontrolled asthma patients on medium-dose ICS/LABA, treatment step-up with add-on LAMA was more likely than step-up to high-dose ICS/LABA in older patients, current smokers, with a history of asthma exacerbations and concomitant diagnosis of COPD.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: F. Lapi and E. Marcona have provided consultations in protocol preparation for epidemiological studies and data analyses for AstraZeneca, GSK and Chiesi. M. Van Der Deijl is an employee of Chiesi. G. Brusselle reports fees for advisory boards and speaker's fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, Merck Sharp & Dohme (MSD) and Sanofi Regeneron. All other authors have nothing to disclose.
Figures



References
-
- World Health Organization . Chronic respiratory diseases. Date last accessed: September 2024. https://www.who.int/health-topics/chronic-respiratory-diseases#tab=tab_1
-
- Global Asthma Network. Global Asthma Report 2018. Date last accessed: September 2024. https://globalasthmareport.org/2018/index.html
-
- Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2019. Date last accessed: March 2024. https://ginasthma.org/wp-content/uploads/2023/04/GINA-Main-Report-2019-W...
LinkOut - more resources
Full Text Sources
Miscellaneous
