ECAP-Controlled Closed-Loop Spinal Cord Stimulation for Chronic Nonsurgical Refractory Back Pain: Subgroup Analysis From Two Prospective Multicenter Clinical Trials
- PMID: 40590186
- PMCID: PMC12594148
- DOI: 10.1097/BRS.0000000000005445
ECAP-Controlled Closed-Loop Spinal Cord Stimulation for Chronic Nonsurgical Refractory Back Pain: Subgroup Analysis From Two Prospective Multicenter Clinical Trials
Abstract
Study design: Subgroup analysis of patients with chronic nonsurgical refractory back pain (NSRBP) from two prospective multicenter clinical trials to 12-month follow-up.
Objective: To evaluate pain-related and holistic response, safety events as well as neurophysiological metrics associated with the use of evoked compound action potential (ECAP)-controlled closed-loop spinal cord stimulation (SCS) for patients with chronic back pain without prior surgery.
Summary of background data: Innovations in SCS such as the development of physiological ECAP-controlled closed-loop SCS overcome limitations of traditional, fixed-output SCS for the treatment of NSRBP. The outcomes of closed-loop SCS to 12-month follow-up for patients with NSRBP have not been previously reported.
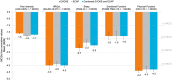
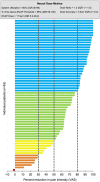
Materials and methods: Patient-reported outcome measures for pain intensity, physical function, health-related quality of life, sleep quality, and emotional function were collected from 68 patients with NSRBP in two prospective multicenter clinical trials. Change in opioid use, its reduction or elimination were assessed at 12-month follow-up. A validated composite outcome measure comprising the different health domains was used to evaluate holistic treatment response through minimal clinically important differences (MCIDs). Objective device metrics provide information on system utilization, loop performance (dose accuracy), and neurophysiological dose metrics.
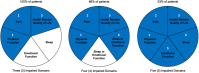
Results: At 12 months, 79% of patients reported ≥50% reduction in pain intensity and 48% obtained ≥80% pain relief. Significant improvements in all patient-reported outcome measures assessed were observed at 3 and 12 months. Voluntary reduction or elimination of opioid use was observed in approximately half of the patients that were taking opioids at baseline. System utilization was >80%, dose ratio was >1.3 ( i.e. 30% above ECAP threshold) with a high-dose accuracy keeping the elicited ECAP within 3.5 μV of the target ECAP set on the system.
Conclusion: ECAP-controlled closed-loop SCS represents a safe and effective treatment option for patients with NSRBP.
Keywords: Persistent Spinal Pain Syndrome Type 1; closed-loop; evoke-compound action potential; neurophysiological dose metrics; nonsurgical refractory back pain; spinal cord stimulation.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
C.W.H. reports consultancy fees from Genecentrix, Saluda Medical, Abbott, and PainTEQ outside the submitted work. J.S.R. reported personal fees from Synergia, Medtronic, BlackRock Neurotech, and Iota outside the submitted work. N.A.M. reports receiving grants from Neuros, Mesoblast, and Vivex Biologics, as well as consulting as a medical monitor for Saluda Medical, Nevro, Vivex Biologics, Mainstay, Sollis Therapeutics, and Vertos outside the submitted work. E.A.P. reports research support from Mainstay, Medtronic, Neuros Medical, Nevro Corp, ReNeuron, SPR, and Saluda Medical outside the submitted work, as well as personal fees from Abbott Neuromodulation, Biotronik, Medtronic Neuromodulation, Nalu, Neuros Medical, Nevro, Presidio Medical, Saluda Medical, and Vertos outside the submitted work. She holds stock options from SynerFuse and neuro42. S.P.L. reports personal fees from Mainstay Medical and Nevro outside the submitted work; he is a consultant for Abbott Laboratories, Boston Scientific, Higgs Boson Health, Medtronic, Minnetronix, Nevro, and Presidio Medical. J.E.P. reports research and consulting fees from Saluda Medical during the conduct of the study; stock options from Saluda Medical; consultancy for Abbott, Medtronic, Saluda Medical, Flowonix, SpineThera, Vertos, Vertiflex, SPR Therapeutics, Tersera, Aurora, Spark, Ethos, Biotronik, Mainstay, WISE, Boston Scientific, and Thermaquil outside the submitted work; has received grant and research support from Abbott, Flowonix, Aurora, PainTEQ, Ethos, Muse, Boston Scientific, SPR Therapeutics, Mainstay, Vertos, AIS, and Thermaquil outside the submitted work; and is a minority shareholder of Vertos, Stimgenics, SPR Therapeutics, Painteq, Aurora, Spark, Celeri Health, Neural Integrative Solutions, Pacific Research Institute, Thermaquil, Abbott and Anesthetic Gas Reclamation. S.J.C. reports receiving grants from Saluda Medical, Vertos, Mainstay, and Vivex outside the submitted work. L.K. reports receiving grants from Nevro, Neuros, Avanos, Medtronic, Neuralace, and Xalud Therapeutics and financial support fromNevro, Avanos, and Saluda Medical outside the submitted work. A.A. reports consultancy fees from Boston Scientific, Stryker, IZI Medical, and Saluda Medical outside the submitted work, sponsored research from Vivex, Boston Scientific, Saluda Medical, and Abbott. R.D.H. reports consultancy for Mainstay Medical, Abbott, Saluda, and Biotronik outside the submitted work and has received research funding from Mainstay Medical, Abbott, Saluda, and Ethos Laboratories. D.S. reports personal fees from Abbott, Boston Scientific, Flowonix, Medtronic, Nevro, Saluda, and Vertiflex outside the submitted work, personal fees and stock options from Mainstay, SPR Therapeutics, PainTEQ, and Vertos, and stock options from Neuralace and Surgentec. S.L. reports consultancy for Avanos, Abbott, Averitas Pharmaceuticals, Biotronik, Nalu, NeuroOne, Nevro (ended), PainTEQ, Presidio, Saluda, SPR Therapeutics, and Vertos outside the submitted work; has received research funding from Avanos, Averitas Pharmaceuticals, Biotronik, Ethos Laboratories (ended), Nalu, Neuralace, Nevro, PainTEQ, Saluda, and SPR Therapeutics; and holds equity in Nalu Medical and NeuroOne. A.M.R. has received research funding from Medtronic, Abbott, and Saluda Medical outside the submitted work. G.L.S. is a clinical advisor for SPR Therapeutics and Saluda Medical. J.H.G. is a consultant for Saluda Medical, Abbott, and Stratus Medical outside the submitted work, and the recipient of research support paid to the institution by SPR Therapeutics and Mainstay Medical. A.L., N.S., and R.V.D. are employees of Saluda Medical. R.V.D. has previously received consultancy fees from Medtronic, and Saluda Medical. T.R.D. is a consultant for Abbott, SpineThera, Saluda Medical, Cornerloc, Boston Scientific, SPR Therapeutics, PainTEQ, Spinal Simplicity, Aurora, Nervonik, and Biotronik outside the submitted work; an advisory board member for Abbott, SPR Therapeutics, Nervonik and Biotronik, has a DRG Lead patent that is pending with Abbott, and has funded research with Abbott, Saluda, Mainstay, SPR Therapeutic, Boston Scientific, and PainTEQ, has stock options with SpineThera, Saluda Medical, PainTEQ, Aurora and Nervonic. The remaning authors report no conflicts of interest.
Figures

References
-
- Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514–530. - PubMed
-
- Itz CJ, Geurts JW, van Kleef M, Nelemans P. Clinical course of non-specific low back pain: a systematic review of prospective cohort studies set in primary care. Eur J Pain. 2013;17:5–15. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

