Immune regeneration: implications for cancer immunotherapy and beyond
- PMID: 40590225
- PMCID: PMC12208541
- DOI: 10.1172/JCI192731
Immune regeneration: implications for cancer immunotherapy and beyond
Abstract
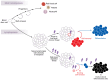
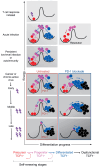
Cancer care is being transformed by therapies leveraging T lymphocytes to attack tumor cells. In parallel, recent basic discoveries have converged into a framework of lymphocyte-dependent immunity as a regenerative process that is sometimes outstripped by high-level engagement. In a stem cell-like fashion, selected T cells must balance mutually opposing demands of differentiation and self-renewal. Activating versus inhibitory signals to T cells instruct opposing cell metabolism, linked to alternative cell fates that arise in sibling cells through lopsided information transfer. Emerging studies indicate that durable immunotherapy response may be limited by the abundance of self-renewing T cells. Leveraging of basic discoveries of regenerative signaling to bolster sustained, stem-like output of freshly differentiated T cells is offering new strategies to overcome cancer immunotherapy resistance. Lymphocyte regeneration may also sustain harmful autoimmune attack. Undercutting the self-renewal of pathogenic clones may thus emerge as a therapeutic strategy for autoimmune diseases.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

