Investigating the Cellular Effects of GALC Dosing in Enzyme Replacement Therapy for Krabbe Disease Supports the Role of Nanomedicine
- PMID: 40590240
- PMCID: PMC12447118
- DOI: 10.1002/adbi.202500147
Investigating the Cellular Effects of GALC Dosing in Enzyme Replacement Therapy for Krabbe Disease Supports the Role of Nanomedicine
Abstract
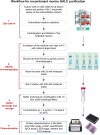
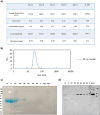
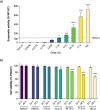
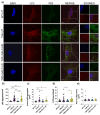
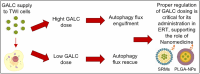
Krabbe disease (KD) is a lysosomal storage disorder characterized by severe neurodegeneration and demyelination. It is caused by mutations in the galactosylceramidase (GALC) gene, leading to the accumulation of psychosine, a neurotoxic metabolite. This study presents an optimized workflow for the production and characterization of recombinant murine GALC (rm-GALC) from HEK293T cells, aiming to improve the feasibility of enzyme replacement therapy (ERT) for KD. An affinity chromatography protocol is refined to purify His-tagged rm-GALC, followed by buffer exchange and concentration steps to produce a stable and active enzyme suitable for subsequent in vitro applications. The purified rm-GALC is characterized for enzymatic activity, purity, and stability using SDS-PAGE, immunoblotting, and dynamic light scattering (DLS). In vitro assays reveal dose-dependent enzymatic activity recovery in KD primary cells upon rm-GALC administration, with no adverse effects on cell viability up to the physiological GALC dose. Additionally, GALC treatment at the physiological dose restored autophagic function in KD cells, as shown by LC3 and p62 marker analyses, confirming its compatibility with lysosomal-autophagic pathways. Conversely, supra-physiological GALC administration resulted in decreased viability and autophagy impairment. Finally, the feasibility of loading GALC into a polymeric nanovector based on stabilized reverse micelles is investigated. These findings highlight the critical importance of precise GALC dose regulation in developing a safe and effective enzyme replacement therapy (ERT) strategy for Krabbe disease (KD), further supporting the potential of a nanovector-mediated ERT approach.
Keywords: Krabbe disease; Twitcher mouse; autophagy modulation; enzyme replacement therapy; eukaryotic recombinant enzymes purification; galactosylceramidase (GALC); globoid cell leukodystrophy; nanomedicine.
© 2025 The Author(s). Advanced Biology published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
- FISA2022-00627/Italian Ministry of University and Research
- 2018-008F2/Preclinical testing of single and combined autophagy modulation by Lithium and Rapamycin in Globoid Cell Leukodystrophy
- 2019-008I2AV1 -/Association Européenne contre les Leucodystrophies
- NanoERT/Association Européenne contre les Leucodystrophies
- Regione Toscana: Bando Ricerca Salute 2018, project DEM AGING
LinkOut - more resources
Full Text Sources
Medical

