Mepolizumab Effectiveness in Severe Asthma With/Without Chronic Rhinosinusitis With Nasal Polyps: Real-World Pooled Analysis
- PMID: 40590259
- PMCID: PMC12444867
- DOI: 10.1111/all.16618
Mepolizumab Effectiveness in Severe Asthma With/Without Chronic Rhinosinusitis With Nasal Polyps: Real-World Pooled Analysis
Abstract
Background: Severe asthma with an eosinophilic phenotype (SAEP) and chronic rhinosinusitis with nasal polyps (CRSwNP) are predominantly type 2-driven diseases, characterised by eosinophilic inflammation and substantial disease burden. Mepolizumab, a humanised monoclonal antibody that targets interleukin-5, a key cytokine in type 2 inflammation, is an effective, approved treatment both in SAEP and CRSwNP. We aimed to analyse real-world evidence of mepolizumab effectiveness in patients with comorbid SAEP and CRSwNP.
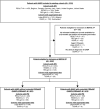
Methods: This study pooled five existing, predominantly European cohorts to describe the impact of mepolizumab on the rate of clinically significant exacerbations (CSEs) and other outcomes in adults with SAEP without and with comorbid CRSwNP (SAEP[-]CRSwNP and SAEP[+]CRSwNP, respectively).
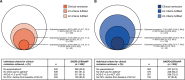
Results: Overall, 1037 patients were included. Baseline characteristics were similar in both cohorts. Mepolizumab was associated with a reduction from baseline in the annual rate of CSEs at 12-months post-initiation (SAEP[-]CRSwNP: 72.7%; SAEP[+]CRSwNP: 79.7%), irrespective of baseline blood eosinophil count (BEC). When patients with SAEP[+]CRSwNP were compared with patients with SAEP[-]CRSwNP, a 30.0% incremental benefit in the reduction of CSEs was observed. At 12-months post-initiation, mepolizumab was also associated with a reduction in oral corticosteroid use and BEC, and an improvement in lung function and Asthma Control Test (ACT) scores in both cohorts. Post-mepolizumab initiation, ≥ 3 clinical remission criteria were fulfilled by 47.2% and 52.3% of patients with SAEP[-]CRSwNP and SAEP[+]CRSwNP, respectively.
Conclusions: The results provide a greater understanding of mepolizumab's effectiveness, demonstrating a substantial improvement in asthma outcomes, irrespective of baseline BEC and the presence of comorbid CRSwNP.
Keywords: asthma; biologics.
© 2025 GSK and The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
Some material discussed herein has been presented at ERS 2024, Vienna, Austria: Palomares I, Loukides S, Schleich F, et al. Eur Resp J 2024; 64 (suppl 68): PA3929:
Figures


References
-
- Domingo C., Sicras‐Mainar A., Sicras‐Navarro A., Sogo A., Mirapeix R. M., and Engroba C., “Prevalence, T2 Biomarkers, and Cost of Severe Asthma in the Era of Biologics: The BRAVO‐1 Study,” Journal of Investigational Allergology & Clinical Immunology 34, no. 2 (2024): 97–105. - PubMed
-
- Heaney L. G., Perez de Llano L., Al‐Ahmad M., et al., “Eosinophilic and Noneosinophilic Asthma: An Expert Consensus Framework to Characterize Phenotypes in a Global Real‐Life Severe Asthma Cohort,” Chest 160, no. 3 (2021): 814–830. - PubMed
-
- Hou R., Ye G., Cheng X., et al., “The Role of Inflammation in Anxiety and Depression in the European U‐BIOPRED Asthma Cohorts,” Brain, Behavior, and Immunity 111 (2023): 249–258. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

