Fabrication of RIG-I-Activating Nanoparticles for Intratumoral Immunotherapy via Flash Nanoprecipitation
- PMID: 40590315
- PMCID: PMC12338309
- DOI: 10.1021/acs.molpharmaceut.5c00125
Fabrication of RIG-I-Activating Nanoparticles for Intratumoral Immunotherapy via Flash Nanoprecipitation
Abstract
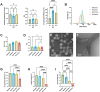
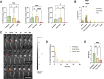
Intratumoral immunotherapy is a promising strategy for stimulating local and systemic antitumor immunity while eliminating or reducing immune-related adverse events often attendant to systemic administration. Activation of the cytosolic pattern recognition receptor retinoic acid-inducible gene I (RIG-I) at tumor sites stimulates innate immunity that can potentiate a T cell-dependent adaptive antitumor immune response. However, the activity and efficacy of 5'-triphosphate RNA (3pRNA) agonists of RIG-I are hindered by poor in vivo stability, rapid degradation, limited cellular uptake, and inefficient cytosolic delivery. To overcome these challenges, we developed RIG-I-activating nanoparticles (RANs) assembled using a flash nanoprecipitation (FNP) process to load a potent stem-loop 3pRNA (SLR) RIG-I agonist into endosome-destabilizing polymeric nanoparticles. We leveraged FNP to induce turbulent micromixing among a corona-forming poly(ethylene glycol)-block-(dimethylaminoethyl methacrylate-co-butyl methacrylate) (PEG-DB) diblock copolymer, a hydrophobic core-forming DB counterpart, and an SLR RIG-I agonist, resulting in the self-assembly of densely loaded nanoparticles that promoted endosomal escape and cytosolic delivery of 3pRNA cargo. Through optimization of polymer properties and inlet feed ratios, we developed RANs with high and improved loading efficiency and increased serum stability relative to a previously reported micelleplex formulation assembled via electrostatic complexation with PEG-DB polymers. We found that optimized RANs exhibited potent immunostimulatory activity in vitro and in vivo when delivered intratumorally. As a result, in preclinical models of MC38 colon cancer and B16.F10 melanoma, intratumoral administration of RANs suppressed tumor growth and increased survival time relative to vehicle controls. Collectively, this work demonstrates that FNP can be harnessed as a versatile and scalable process for the efficient loading of nucleic acids into polymeric nanoparticles and highlights the potential of RANs as a translationally promising platform for intralesional cancer immunotherapy.
Keywords: RIG-I; cancer immunotherapy; drug delivery; flash nanoprecipitation; nanoparticle; polymer.
Figures



Similar articles
-
Nanoparticle Retinoic Acid-Inducible Gene I Agonist for Cancer Immunotherapy.ACS Nano. 2024 May 7;18(18):11631-11643. doi: 10.1021/acsnano.3c06225. Epub 2024 Apr 23. ACS Nano. 2024. PMID: 38652829 Free PMC article.
-
The Human Papillomavirus E6 Oncoprotein Targets USP15 and TRIM25 To Suppress RIG-I-Mediated Innate Immune Signaling.J Virol. 2018 Feb 26;92(6):e01737-17. doi: 10.1128/JVI.01737-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29263274 Free PMC article.
-
Targeting Intracellular Innate RNA-Sensing Systems Overcomes Resistance to CAR T-cell Therapy in Solid Tumors.Cancer Res. 2025 Jul 15;85(14):2679-2693. doi: 10.1158/0008-5472.CAN-24-3425. Cancer Res. 2025. PMID: 40305099 Free PMC article.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Nicotine receptor partial agonists for smoking cessation.Cochrane Database Syst Rev. 2023 May 5;5(5):CD006103. doi: 10.1002/14651858.CD006103.pub8. Cochrane Database Syst Rev. 2023. PMID: 37142273 Free PMC article.
References
-
- Kelly C. M., Antonescu C. R., Bowler T., Munhoz R., Chi P., Dickson M. A., Gounder M. M., Keohan M. L., Movva S., Dholakia R., Ahmad H., Biniakewitz M., Condy M., Phelan H., Callahan M., Wong P., Singer S., Ariyan C., Bartlett E. K., Crago A., Yoon S., Hwang S., Erinjeri J. P., Qin L.-X., Tap W. D., D’Angelo S. P.. Objective Response Rate Among Patients With Locally Advanced or Metastatic Sarcoma Treated With Talimogene Laherparepvec in Combination With Pembrolizumab. JAMA Oncol. 2020;6(3):402. doi: 10.1001/jamaoncol.2019.6152. - DOI - PMC - PubMed
-
- Toulmonde M., Cousin S., Kind M., Guegan J.-P., Bessede A., Le Loarer F., Perret R., Cantarel C., Bellera C., Italiano A.. Randomized Phase 2 Trial of Intravenous Oncolytic Virus JX-594 Combined with Low-Dose Cyclophosphamide in Patients with Advanced Soft-Tissue Sarcoma. J. Hematol Oncol. 2022;15(1):149. doi: 10.1186/s13045-022-01370-9. - DOI - PMC - PubMed
-
- Samson A., West E. J., Carmichael J., Scott K. J., Turnbull S., Kuszlewicz B., Dave R. V., Peckham-Cooper A., Tidswell E., Kingston J., Johnpulle M., da Silva B., Jennings V. A., Bendjama K., Stojkowitz N., Lusky M., Prasad K. R., Toogood G. J., Auer R., Bell J., Twelves C. J., Harrington K. J., Vile R. G., Pandha H., Errington-Mais F., Ralph C., Newton D. J., Anthoney A., Melcher A. A., Collinson F.. Neoadjuvant Intravenous Oncolytic Vaccinia Virus Therapy Promotes Anticancer Immunity in Patients. Cancer Immunol Res. 2022;10(6):745–756. doi: 10.1158/2326-6066.CIR-21-0171. - DOI - PMC - PubMed
-
- Mahalingam D., Wilkinson G. A., Eng K. H., Fields P., Raber P., Moseley J. L., Cheetham K., Coffey M., Nuovo G., Kalinski P., Zhang B., Arora S. P., Fountzilas C.. Pembrolizumab in Combination with the Oncolytic Virus Pelareorep and Chemotherapy in Patients with Advanced Pancreatic Adenocarcinoma: A Phase Ib Study. Clin. Cancer Res. 2020;26(1):71–81. doi: 10.1158/1078-0432.CCR-19-2078. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

