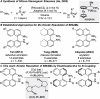
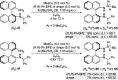
Kinetic Resolution of BINAMs by Stereoselective Copper-Catalyzed Dehydrogenative Si-N Coupling with Prochiral Dihydrosilanes
- PMID: 40590348
- PMCID: PMC12261316
- DOI: 10.1021/acs.orglett.5c02258
Kinetic Resolution of BINAMs by Stereoselective Copper-Catalyzed Dehydrogenative Si-N Coupling with Prochiral Dihydrosilanes
Abstract
A nonenzymatic kinetic resolution of monoprotected 1,1'-binaphthyl-2,2'-diamine (BINAM) derivatives is reported. This is achieved by a Cu-H-catalyzed dehydrogenative Si-N coupling with prochiral dihydrosilanes using (R,R)-Ph-BPE as a chiral ligand. The atroposelective as well as diastereoselective N-silylation enables the resolution of BINAMs with various substituents in 6,6'- or 7,7'-positions with good to synthetically useful selectivity factors.
Figures
Similar articles
-
Kinetic Resolution of BINOLs and Biphenols by Atroposelective, Cu-H-Catalyzed Si-O Coupling with Hydrosilanes.Org Lett. 2024 Nov 8;26(44):9531-9535. doi: 10.1021/acs.orglett.4c03557. Epub 2024 Oct 24. Org Lett. 2024. PMID: 39448059 Free PMC article.
-
Naphthylpyridyl-Cu(II)-Catalyzed Oxidative Coupling of 2-Naphthylamines to Access BINAM Derivatives.Org Lett. 2025 Aug 8. doi: 10.1021/acs.orglett.5c02740. Online ahead of print. Org Lett. 2025. PMID: 40779713
-
Emerging Capabilities of Nonclassical Noncovalent Interactions and Asymmetric Catalysis in Stereoselective Glycosylations and Carbohydrate Functionalizations.Acc Chem Res. 2025 Jul 1;58(13):2124-2144. doi: 10.1021/acs.accounts.5c00289. Epub 2025 Jun 12. Acc Chem Res. 2025. PMID: 40503847 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
References
-
- Isobe T., Fukuda K., Araki Y., Ishikawa T.. Modified Guanidines as Chiral Superbases: The First Example of Asymmetric Silylation of Secondary Alcohols. Chem. Commun. 2021:243–244. doi: 10.1039/b009173l. - DOI
-
- Hoveyda, A. H. ; Snapper, M. L. . Enantioselective Synthesis of Silyl Ethers Through Catalytic Si–O Bond Formation. In Organosilicon Chemistry: Novel Approaches and Reactions; Hiyama, T. , Oestreich, M. , Eds.; Wiley-VCH: 2019; pp 459–493 and references cited therein.
LinkOut - more resources
Full Text Sources