SARS-CoV-2 RNA-binding protein suppresses extracellular miRNA release
- PMID: 40590376
- PMCID: PMC12239786
- DOI: 10.1080/15476286.2025.2527494
SARS-CoV-2 RNA-binding protein suppresses extracellular miRNA release
Abstract
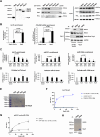
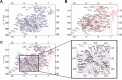
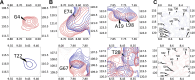
SARS-CoV-2 is the betacoronavirus causing the COVID-19 pandemic. Although the SARS-CoV-2 genome and transcriptome were reported previously, the function of individual viral proteins is largely unknown. Utilizing biochemical and molecular biology methods, we identified that four SARS-CoV-2 RNA-binding proteins (RBPs) regulate the host RNA metabolism by direct interaction with mature miRNA let-7b revealed by Nuclear Magnetic Resonance spectroscopy (NMR). SARS-CoV-2 RBP Nsp9 primarily binds mature miRNA let-7b, a direct ligand of the Toll-like Receptor 7 (TLR7), one of the potential SARS-CoV-2 therapeutics. Nsp9 suppresses host gene expression possibly by promoting let-7b-mediated silencing of a cellular RNA polymerase, POLR2D. In addition, Nsp9 inhibits extracellular release of let-7b and subsequent antiviral activity via TLR7. These results demonstrate that SARS-CoV-2 hijacks the host RNA metabolism to suppress antiviral responses and to shut down cellular transcription. Our findings of how a natural ligand of TLR7, miRNA let-7b, is suppressed by SARS-CoV-2 RBPs will advance our understanding of COVID-19 and SARS-CoV-2 therapeutics.
Keywords: Nsp9; POLR2D; SARS-CoV-2; let-7b; miRNA.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
