Registration and Real-Life Studies on Automated Insulin Delivery Systems
- PMID: 40590465
- PMCID: PMC12213531
- DOI: 10.1177/19322968251334993
Registration and Real-Life Studies on Automated Insulin Delivery Systems
Abstract
Introduction: The Diabetes Control and Complications Trial (DCCT) clearly documented long-term beneficial effects on both micro- and macro-vascular complications associated with type 1 diabetes (T1D) by using intensive insulin therapy (IIT) via multiple daily injections (MDIs) or insulin pumps more than 30 year ago. IIT, both during the DCCT and with translation into clinical practice, has been demonstrated to increase the risk of severe hypoglycemia and weight gain. Automated insulin delivery (AID) systems have become the standard of care in T1D management in the developed countries.
Materials and methods: We reviewed the registration and real-life studies for different AID systems reported to date. Many of the registration studies were sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). A systematic literature search was conducted using the MEDLINE (PubMed) database. Studies with the longest duration and/or with the largest number of participants were included.
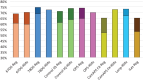
Results: In the last decade, the introduction of a many AID systems for patients with T1D has shown improvements in glycemic metrics as documented by HbA1c values, time in range (TIR), time below range (TBR), and quality of life. Most of the registration and real-life studies have shown safe and effective use of AID systems for all age groups living with T1D.
Conclusions: In this review, we summarize the registration and real-life studies of US Food and Drug Administration (FDA)-approved AID systems. Real-life studies confirmed the glycemic outcomes of AID systems reported from registration studies.
Keywords: CGM; CSII; HbA1c; artificial pancreas; hyperglycemia; hypoglycemia; insulin pump; time below range; time in range.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: H.K.A. reports research support and honorarium through the University of Colorado from Medtronic, Dexcom, Roche, and Tandem Diabetes Care. S.K.G. has served on Advisory Boards and received consulting fees from Medtronic, Eli Lilly, and Novo Nordisk. Through the University of Colorado, he has received research grants from Eli Lilly, Medtronic, Dario, Lexicon, NCI, and Dexcom. B.P.K. reports research grants handled by the University of Virginia from the National Institutes of Health, Novo Nordisk, Dexcom, and Tandem Diabetes Care. In addition, B.P.K. has a number of patents with royalties paid to Dexcom and Novo Nordisk, outside of the submitted study. R.V. is a full-time employee of Medtronic Diabetes. R.H. reports having received speaker honoraria from Eli Lilly, Dexcom, and Novo Nordisk; receiving license fees from Braun; receiving consultancy fees from Abbott Diabetes Care; patents related to closed-loop; and being director at CamDiab. R.W.B. reports no personal financial disclosures but reports that his institution has received funding on his behalf as follows: grant funding, study supplies, and consulting fees from Insulet, Tandem Diabetes Care, and Beta Bionics; grant funding and study supplies from Dexcom; grant funding from Bigfoot Biomedical; study supplies from Medtronic, Ascencia, and Roche; consulting fees and study supplies from Eli Lilly and Novo Nordisk; and consulting fees from embecta, Sequel Med Tech, Vertex, Hagar, Ypsomed, Sanofi, and Zucara. M.D.B. reports research grants handled by the University of Virginia from the National Institutes of Health, Novo Nordisk, and Dexcom, and nonfinancial research support from Tandem Diabetes Care, outside of the submitted study. In addition, M.D.B. has a number of patents with royalties licensed to Dexcom, Sanofi, Tandem Diabetes Care, and Novo Nordisk, outside of the submitted study. M.D.B. finally reports consulting/speakership activities with Dexcom, Tandem Diabetes Care, Roche, Portal Insulin LLC, BoydSense, and Vertex. S.A.B. has received research support through her institution from Dexcom, Insulet, Roche Diagnostics, Tandem Diabetes Care, and Tolerion and has participated on a data monitoring board for MannKind. J.L.S. has received grants or contracts from Breakthrough T1D, the National Institute of Diabetes and Digestive and Kidney Diseases, Jaeb Center for Health Research, Insulet, Medtronic, Provention Bio, and Abbott paid to her institution; has received consulting fees from Abbott; has received payment or honoraria from Insulet, Medtronic Diabetes, and Zealand Pharma; has participated in advisory boards for Bigfoot Biomedical, Cecelia Health, Insulet, Medtronic Diabetes, Novo Nordisk, and Vertex Pharmaceuticals; and reports owning stock/stock options of StartUp Health T1D Moonshot. R.M.B. has received research support, has acted as a consultant, or has been on the scientific advisory board for Abbott Diabetes Care, Ascensia, CeQur, DexCom, Eli Lilly, Embecta, Hygieia, Insulet, Medscape, Medtronic, Novo Nordisk, Onduo, Roche Diabetes Care, Tandem Diabetes Care, Sanofi, United Healthcare, Vertex Pharmaceuticals, and Zealand Pharma. D.C.K is consultant FT for Afon, Glucotrack, Lifecare, Novo, Samsung, Thirdwayv, and Tingo.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

