Deciphering cell cycle organization of Toxoplasma endodyogeny
- PMID: 40590555
- PMCID: PMC12345243
- DOI: 10.1128/mbio.01119-25
Deciphering cell cycle organization of Toxoplasma endodyogeny
Abstract
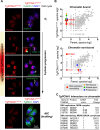
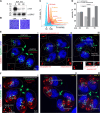
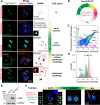
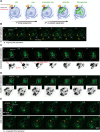
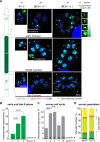
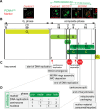
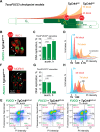
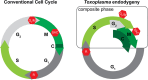
In this study, we report the atypical cell cycle organization of the unicellular eukaryotic pathogen Toxoplasma gondii. The remarkably flexible cell division of T. gondii and other apicomplexan parasites differs considerably from the cell division modes employed by other model eukaryotes. In addition, there is a lack of recognizable cell cycle regulators, which has contributed to the difficulties in deciphering the order of events in the apicomplexan cell cycle. To aid in studies of the cell cycle organization of the T. gondii tachyzoite, we have created the Fluorescent Ubiquitination-based Cell Cycle Indicator probes, ToxoFUCCIS and ToxoFUCCISC. We introduced a DNA replication factor TgPCNA1 tagged with NeonGreen that can be used alone or in conjunction with an mCherry-tagged budding indicator TgIMC3 in the auxin-induced degradation parental strain. The varied localization and dynamic cell cycle oscillation have confirmed TgPCNA1 to be a suitable T. gondii FUCCI probe. The ToxoFUCCIS analysis showed that tachyzoite DNA replication starts at or near centromeric regions and has a bell-shaped dynamic and a significant degree of the cell cycle asynchrony within the vacuoles. Quantitative live and immunofluorescence microscopy analyses of ToxoFUCCIS and its derivatives co-expressing epitope-tagged cell cycle markers have revealed an unusual composite cell cycle phase that incorporates overlapping S, G2, mitosis, and cytokinesis (budding). We identified five intervals of the composite phase and their approximate duration: S (19%), S/G2/C (3%), S/M/C (9%), M/C (18%), and C/G1 (<1%). The ToxoFUCCIS probe efficiently detected G2/M and Spindle Assembly Checkpoints, as well as the SB505124-induced TgMAPK1-dependent block. Altogether, our findings showed an unprecedented complexity of the cell cycle in apicomplexan parasites.
Importance: The cell division rates directly correlate with the severity of the diseases caused by apicomplexan parasites. Despite its clinical importance, little is known about the apicomplexan cell cycle that controls parasite division rates. Previous studies implied that the apicomplexan cell cycle is organized differently from the cell cycle of their host cells. However, the order of cell cycle events had never been established. In the current study, we present evidence of the highly unusual organization of the Toxoplasma gondii cell cycle. Using a new cell cycle indicator, we measured the duration of individual cell cycle processes in Toxoplasma tachyzoites and revealed the unprecedented overlaps of four cell cycle phases. Our findings explain how the apicomplexan cell cycle accommodates the flexibility of the division modes and identify unique steps of the parasite survival program that can be explored in the future.
Keywords: Apicomplexa; Cdk-related kinase; FUCCI; PCNA1; Toxoplasma gondii; cell cycle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

