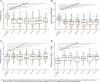
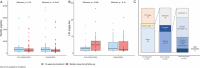
Luspatercept for Transfusion-Dependent Beta-Thalassemia: Real-World Experience in a Large Cohort of Patients From Italy
- PMID: 40590626
- PMCID: PMC12326213
- DOI: 10.1002/ajh.27758
Luspatercept for Transfusion-Dependent Beta-Thalassemia: Real-World Experience in a Large Cohort of Patients From Italy
Abstract
Keywords: extramedullary hematopoiesis; luspatercept; real‐world setting; thromboembolism; transfusion‐dependent thalassemia.
Conflict of interest statement
R.O. has received speaker honoraria from BMS, Vertex, and Chiesi and received consultancy fees from BMS, Vertex, and Agios. V.M.P. was a member of the advisory board, part of the speaker's bureau for BMS, and a consultant for Vertex. F.L. was a consultant, a member of the advisory board, part of the speaker's bureau, and participated in educational events for BMS and Vertex. I.M. was a member of the advisory board of BMS, Vertex, and Sanofi, received honoraria for lectures from Bristol Myers Squibb and Sanofi, and research support from Sanofi. M.D.C. was a member of the advisory board for Sanofi Genzyme, BMS/Celgene, Vertex/CRISPR, Novo Nordisk, Pharmacosmos, and Agios. P.R. received honoraria from Agios Pharmaceuticals and BMS (Celgene) and was a member of the safety monitoring board/advisory board for Agios Pharmaceuticals and BMS (Celgene). R.R. received speaker honoraria and research support from Vertex and BMS. The other authors declare no conflicts of interest.
Figures
References
-
- Cappellini M. D., Viprakasit V., Georgiev P., et al., “Long‐Term Efficacy and Safety of Luspatercept for the Treatment of Anaemia in Patients With Transfusion‐Dependent β‐Thalassaemia (BELIEVE): Final Results From a Phase 3 Randomised Trial,” Lancet Haematology 12, no. 3 (2025): e180–e189, 10.1016/S2352-3026(24)00376-4. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

