Tocilizumab prophylaxis for patients with multiple myeloma treated with bispecific antibodies
- PMID: 40590849
- PMCID: PMC12514479
- DOI: 10.1182/bloodadvances.2025016911
Tocilizumab prophylaxis for patients with multiple myeloma treated with bispecific antibodies
Abstract
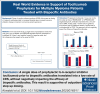
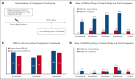
Bispecific antibodies for treatment for multiple myeloma are highly effective but commonly cause cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). Emerging data indicate that prophylactic tocilizumab may reduce CRS, without impacting efficacy. We administered a single dose of tocilizumab before the first dose of bispecific antibodies to 119 patients to determine the impact on CRS in a real-world setting including B-cell maturation antigen × CD3- and G-protein-coupled receptor class C group 5 member D × CD3-targeted antibodies. The best overall response rate was 65.7% (binomial 95% confidence interval [CI], 55.8-74.7). We observed a low overall rate of CRS (10.1%; 95% CI, 5.3-17). For teclistamab, elranatamab, linvoseltamab, and talquetamab individually, the CRS rate was 8.9%, 12.5%, 0%, and 13%, respectively. The overall rate of ICANS (5.9%; 95% CI, 2.4-11.7) was low but similar to rates without prophylactic tocilizumab. CRS was limited to grade 1 for 10 of 12 events. There were no grade 3 CRS events, and no additional doses of tocilizumab or corticosteroids were given for CRS. Our real-world evidence results suggest that tocilizumab may be effective as a preventive, rather than reactive, measure to prevent CRS without compromising efficacy.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: O.L. reports funding from National Cancer Institute/National Institutes of Health, US Food and Drug Administration, Leukemia and Lymphoma Society, Rising Tide Foundation, Multiple Myeloma Research Foundation, International Myeloma Foundation, Paula and Rodger Riney Foundation, Tow Foundation, Perelman Family Foundation, Myeloma Solutions Fund, Cannon Guzy Family Fund, Amgen, Celgene, Janssen, Takeda, Glenmark, Seattle Genetics, and Karyopharm; honoraria from AbbVie, Adaptive, Amgen, Binding Site, Bristol Myers Squibb, Celgene, Cellectis, GlaxoSmithKline (GSK), Janssen, Juno, and Pfizer; participated on advisory boards for AbbVie, Adaptive, Amgen, Binding Site, Bristol Myers Squibb, Celgene, Cellectis, GSK, Janssen, Juno, and Pfizer; and served on independent data monitoring committees for international randomized trials by Takeda, Merck, Janssen, and Novartis outside the submitted work. The remaining authors declare no competing financial interests.
Figures
References
-
- Rodriguez-Otero P, Usmani S, Cohen AD, et al. International Myeloma Working Group immunotherapy committee consensus guidelines and recommendations for optimal use of T-cell-engaging bispecific antibodies in multiple myeloma. Lancet Oncol. 2024;25(5):e205–e216. - PubMed
-
- Sharma A, Singh V, Deol A. Epidemiology and predictors of 30-day readmission in CAR-T cell therapy recipients. Transpl Cell Ther. 2023;29(2):108.e1–108.e7. - PubMed
-
- Niels WCJ van de Donk, Alfred L Garfall, Lotfi Benboubker, Katarina Uttervall . ASCO Annual Meeting; Chicago, IL: 31 May to 4 June 2024. Longer-term follow-up of patients (pts) receiving prophylactic tocilizumab (toci) for the reduction of cytokine release syndrome (CRS) in the phase 1/2 MajesTEC-1 study of teclistamab in relapsed/refractory multiple myeloma (RRMM). Paper presented at. 2024.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials