Refinement of day 28 treatment response criteria for acute GVHD: a collaboration study of the JSTCT and MAGIC
- PMID: 40590886
- PMCID: PMC12496244
- DOI: 10.1182/bloodadvances.2025016233
Refinement of day 28 treatment response criteria for acute GVHD: a collaboration study of the JSTCT and MAGIC
Abstract
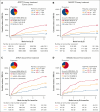
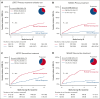
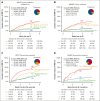
Overall response (OR) that combines complete (CR) and partial responses (PR) is the conventional end point for acute graft-versus-host disease (GVHD) trials. Because PR includes heterogeneous clinical presentations, reclassifying PR could produce a better end point. Patients in the primary treatment cohort from the Japanese Society for Transplantation and Cellular Therapy (JSTCT) were randomly divided into training and validation sets. In the training set, a classification and regression tree algorithm generated day 28 refined response (RR) criteria based on symptoms at treatment and day 28. We then evaluated RR for primary and second-line treatments, using the area under the receiver operating characteristic curve (AUC) and negative predictive value (NPV) for 6-month nonrelapse mortality as performance measures. RR considered patients with grade 0/1 at day 28 without additional treatment as responders. RR for primary treatment produced higher AUCs than OR with small improvement of NPVs in both validation sets: JSTCT (AUC, 0.73 vs 0.69 [P < .001]; NPV, 92.0% vs 89.6% [P < .001]) and the Mount Sinai Acute GVHD International Consortium (MAGIC; AUC, 0.71 vs 0.68 [P = .032]; NPV, 90.9% vs 89.8% [P = .009]). RR for second-line treatment produced similar AUCs but much higher NPVs than OR in both validation sets of JSTCT (AUC, 0.64 vs 0.63 [P = .775]; NPV, 74.5% vs 66.0% [P < .001]) and MAGIC (AUC, 0.67 vs 0.64 [P = .105]; NPV, 86.8% vs 76.1% [P = .004]). Classifying persistent but mild skin symptoms as responses and residual lower gastrointestinal GVHD as nonresponses were major drivers in improving the prognostic performance of RR. Our externally validated day 28 RR would serve as a better end point than conventional criteria in future first- and second-line treatment trials.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: Y. Akahoshi reports honoraria from Novartis and AstraZeneca. Y.I. reports honoraria from Meiji Seika Pharma, Novartis, and Janssen Pharmaceutical K.K.; and research grants from Meiji Seika Pharma, Incyte, and Amgen. H.N. reports honoraria from Merck Sharp and Dohme (MSD), Otsuka Pharmaceutical, Pfizer, Novartis, Takeda Pharmaceutical, Janssen Pharmaceutical, Chugai Pharmaceutical, Sanofi, Meiji Seika Pharma, Asahi Kasei Pharma, and Nippon Shinyaku; and research funding from JCR Pharmaceuticals, Kyowa Kirin, Taiho Pharma, Santen Pharmaceutical, and Terumo. N.A. reports speakers’ bureau fees/honoraria from AbbVie, Nippon Shinyaku, Meiji Seika Pharma, Otsuka Pharmaceutical, Daiichi Sankyo, Novartis, Kyowa Kirin, Astellas, Asahi Kasei Pharma, BeiGene, and Novartis; and research funding from Novartis. M.A.D. reports consultancy fees from Comanche Biopharma and Bloomer Tech. F.A. reports honoraria and consultancy fees from Bristol Myers Squibb, Medac, Novartis, Miltenyi Biomedicine, Janssen Pharmaceutical, Kite, AbbVie, and Mallinckrodt/Therakos; and research funding from Mallinckrodt/Therakos. H.K.C. reports consultancy fees from Ironwood Pharmaceuticals, Actinium, AbbVie, REGiMMUNE, Sanofi, Orca Bio, and Incyte. A.M.E. has consulted for Incyte (advisory board). E.O.H. reports consultancy fees from Disc Medicine. K.K. reports honoraria from AbbVie, Pfizer, Nippon Shinyaku, Meiji Seika Pharma, Otsuka Pharmaceutical, Daiichi Sankyo, Celgene, Novartis, AstraZeneca, Ono Pharmaceutical, Kyowa Kirin, Sumitomo Dainippon Pharma, SymBio Pharmaceuticals, Sanofi, Alexion Pharmaceuticals, Bristol Myers Squibb, and Janssen Pharmaceutical; research funding from Shionogi, Otsuka Pharmaceutical, JCR Pharmaceuticals, Takeda Pharmaceutical, Japan Blood Products Organization, Mochida Pharmaceutical, Asahi Kasei Pharma, Chordia Therapeutics, Chugai Pharmaceutical, Teijin Pharma, Eisai, and Kyowa Kirin; and is a current equity holder in Asahi Genomics. M.T. reports honoraria from AbbVie, Kyowa Kirin, Daiichi Sankyo, Sumitomo Pharma, Astellas Pharma, Pfizer, Otsuka Pharmaceutical, MSD, Asahi Kasei Pharma, Chugai Pharmaceutical, Amgen, Janssen Pharmaceutical, Nippon Shinyaku, Novartis, and Meiji Seika Pharma; and research funding from Chugai Pharmaceutical. N.U. has received honoraria from CSL Behring, MSD, Astellas Pharma, AstraZeneca, AbbVie, Otsuka Pharmaceutical, Kyowa Kirin, SymBio Pharmaceuticals, Daiichi Sankyo, Takeda Pharmaceutical, and Novartis; research funding from Chugai Pharmaceutical, Fuji Pharma, Nippon Boehringer Ingelheim, JCR Pharmaceuticals, and Sumitomo Pharma; and consultancy fees from Takeda Pharmaceutical. S.Y. has received honoraria from Daiichi Sankyo, Novartis, Genmab, Janssen, Pfizer, Asahi Kasei Pharma, Meiji Seika Pharma, Takeda, Gilead, MSD, Bristol Myers Squibb, Sanofi, AbbVie, Chugai, AstraZeneca, and Ono Pharmaceutical. Y.-B.C. reports consultancy fees from Ironwood Pharmaceuticals, Vor Bio, Garuda, Editas, Alexion, and Incyte. J.K. reports honoraria from Janssen Pharmaceutical, Astellas Pharma, CSL Behring, MSD, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, Chugai Pharmaceutical, Amgen, Otsuka Pharmaceutical, Bristol Myers Squibb, Ono Pharmaceutical, Asahi Kasei Pharma, Sanofi, CareNet, Inc, Kyowa Kirin, Nippon Shinyaku, Nippon Kayaku, Novartis, Daiichi Sankyo, and AbbVie; consultancy fees from Janssen Pharmaceutical, Astellas Pharma, Novartis, Daiichi Sankyo, Megakaryon, SymBio Pharmaceuticals, and AbbVie; and research funding from Eisai. R.N. reports research funding from Mitarisan, Helocyte, and MaaT Pharma; and consultancy fees from Omeros, bluebird bio, Sanofi, Ono Pharmaceutical, and Pfizer. Y. Atsuta reports speakers’ bureau fees/honoraria from Otsuka Pharmaceutical, Chugai Pharmaceutical, Novartis Pharma K.K., Meiji Seika Pharma, and Janssen Pharmaceutical K.K.; and consultancy fees from JCR Pharmaceuticals. J.E.L. reports research support from Equillium, Incyte, MaaT Pharma, and Mesoblast; and consulting fees from Sanofi, bluebird bio, Inhibrx, X4 Pharmaceuticals, Editas, Equillium, Kamada, and Mesoblast. J.L.M.F. reports consulting fees from Editas, Equillium, Kamada, Mesoblast, Alexion, Realta, Medpace, Viracor, AlloVir, and Physicians’ Education Resource; and research support from Equillium, Incyte, MaaT Pharma, and Mesoblast. Y.K. reports honoraria from Asahi Kasei, MSD, Novartis, Pfizer, Sanofi, Chugai, Astellas, and Kyowa Kirin; and research funding from Chugai, Kyowa Kirin, Asahi Kasei, and Otsuka. T.T. reports honoraria from Nippon Shinyaku, Daiichi Sankyo, Novartis, Otsuka, Genmab, Janssen, Pfizer, Kyowa Kirin, Asahi Kasei Pharma, Meiji Seika Pharma, Takeda, Gilead, SymBio Pharmaceuticals, MSD, Bristol Myers Squibb, Sanofi, Nippon Kayaku, AbbVie, Chugai, AstraZeneca, and Astellas; consultancy fees from Kyowa Kirin, Meiji Seika Pharma, Takeda, Roche Diagnostics, and Nippon Shinyaku; and research funding from Daiichi Sankyo, Otsuka, Kyowa Kirin, Asahi Kasei Pharma, LUCA Science, PharmaEssentia Japan, Sumitomo Pharma, JCR Pharmaceuticals, Chugai, and Astellas. The remaining authors declare no competing financial interests.
Figures


References
-
- Penack O, Marchetti M, Aljurf M, et al. Prophylaxis and management of graft-versus-host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2024;11(2):e147–e159. - PubMed
-
- Jamy O, Zeiser R, Chen YB. Novel developments in the prophylaxis and treatment of acute GVHD. Blood. 2023;142(12):1037–1046. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

