Insight into NeuroCOVID: neurofilament light chain (NfL) as a biomarker in post-COVID-19 patients with olfactory dysfunctions
- PMID: 40591023
- PMCID: PMC12213847
- DOI: 10.1007/s00415-025-13222-w
Insight into NeuroCOVID: neurofilament light chain (NfL) as a biomarker in post-COVID-19 patients with olfactory dysfunctions
Abstract
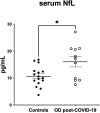
The term NeuroCOVID was coined to describe the neurological consequences observed in COVID-19 patients. Numerous patients infected with SARS-CoV-2 reported olfactory dysfunction as the first symptom preceding clinical manifestations, such as cough and fever, or even the only symptom, suggesting the sudden loss of smell or hyposmia as an important predictive factor for COVID-19 infection. Several patients developed long-term olfactory impairment, but to date there is not available a biochemical diagnosis of anosmia. The aim of this pilot study is to investigate the association between neurofilament light-chain (NfL) serum levels and the olfactory dysfunctions in post-COVID-19 patients. This study recruited patients who developed COVID-19 between January 2020 and August 2021. They were evaluated between October 2022 and March 2023 by Sniffin' Sticks tests to investigate deficits of odor identification, discrimination, and threshold and serum NfL biomarker measurement to assess a neuronal damage. Out of 27 patients, 11 were affected by post-viral permanent olfactory dysfunction (named Od-post-COVID-19) and 16 healed from the infection without residual Od problem, as a control group. We observed an increased levels of NfL 16.02 ± 1.91 pg/mL in Od-post-COVID-19, suggesting that NfL to be recognized as a biomarker of post-viral olfactory dysfunction, supporting the diagnostic process of NeuroCOVID, joined with other well-known neurological biomarkers and/or innovative investigative approaches.
Keywords: Anosmia; COVID-19; Long COVID; Nasal mucosa; Neuro-olfactory epithelium; NeuroCOVID; Neurofilament light chain (NfL); Olfactory dysfunction.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflicts of interest: The authors declare no conflicts of interest. Institutional review board statement: The study was conducted according to the Declaration of Helsinki and followed the Institutional Review Board standards of the Sapienza University of Rome, Policlinico Umberto I (ethical committee Ref. 6536). Informed consent: Informed consent was obtained from all subjects involved in the study.
Figures
Similar articles
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Olfaction and Plasma Biomarkers of Alzheimer Disease and Neurodegeneration in the Atherosclerosis Risk in Communities Study.Neurology. 2025 Jun 10;104(11):e213706. doi: 10.1212/WNL.0000000000213706. Epub 2025 May 15. Neurology. 2025. PMID: 40373252
-
Pathophysiological relationship between COVID-19 and olfactory dysfunction: A systematic review.Braz J Otorhinolaryngol. 2022 Sep-Oct;88(5):794-802. doi: 10.1016/j.bjorl.2021.04.001. Epub 2021 Apr 25. Braz J Otorhinolaryngol. 2022. PMID: 33965353 Free PMC article.
-
Intranasal insulin for COVID-19-related smell loss.Eur Arch Otorhinolaryngol. 2024 Jan;281(1):201-205. doi: 10.1007/s00405-023-08176-6. Epub 2023 Aug 22. Eur Arch Otorhinolaryngol. 2024. PMID: 37608216
-
Platelet-Rich Plasma for Treating COVID-19-Related Anosmia, Hyposmia, and Parosmia: A Controlled Longitudinal Study.Otolaryngol Head Neck Surg. 2025 Apr;172(4):1450-1458. doi: 10.1002/ohn.1149. Epub 2025 Jan 31. Otolaryngol Head Neck Surg. 2025. PMID: 39888025 Clinical Trial.
References
-
- World Health Organization (2023) data.who.int, WHO Coronavirus (COVID-19) dashboard > About [Dashboard]. https://data.who.int/dashboards/covid19/cases?n=o
-
- Groff D, Sun A, Ssentongo AE, Ba DM, Parsons N, Poudel GR, Lekoubou A, Oh JS, Ericson JE, Ssentongo P, Chinchilli VM (2021) Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open 4(10):e2128568. 10.1001/jamanetworkopen.2021.28568 - PMC - PubMed
-
- Bozzetti S, Ferrari S, Zanzoni S, Alberti D, Braggio M, Carta S, Piraino F, Gabbiani D, Girelli D, Nocini R, Monaco S, Crisafulli E, Mariotto S (2021) Neurological symptoms and axonal damage in COVID-19 survivors: Are there sequelae? Immunol Res 69(6):553–557. 10.1007/s12026-021-09220-5 - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous