Neoadjuvant treatment patterns and biomarker selection in muscle-invasive bladder cancer
- PMID: 40591027
- PMCID: PMC12214084
- DOI: 10.1007/s12672-025-02796-6
Neoadjuvant treatment patterns and biomarker selection in muscle-invasive bladder cancer
Abstract
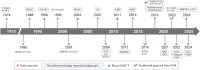
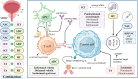
As the feasibility of risk-adaptive bladder-sparing treatment is increasingly validated, the prospects for neoadjuvant therapy in muscle-invasive bladder cancer (MIBC) are rapidly evolving. For patients seeking effective and tolerable treatment options, platinum-based chemotherapy, particularly dose-dense MVAC (ddMVAC), remains the preferred standard. However, the emergence of novel interventions such as immune checkpoint inhibitors (ICIs), FGFR inhibitors, and antibody-drug conjugates (ADCs) offers promising alternatives, especially for those ineligible for cisplatin-based regimens. Ongoing clinical trials, including KEYNOTE-B15, RC48-C017, and NIAGARA, are actively investigating the efficacy of combining these agents with existing neoadjuvant therapies, aiming to establish new first-line treatment options. Although predictive models based on histological features, DNA damage repair (DDR) genes, molecular subtyping, liquid biopsies, and in vitro organoids have demonstrated potential in guiding treatment selection, the clinical translation process remains slow. There is a pressing need to accelerate the exploration of genetic heterogeneity in MIBC and to validate the clinical utility of emerging biomarkers to optimize patient selection for neoadjuvant therapy. This review will comprehensively examine the evolution of neoadjuvant treatment paradigms, focusing on high-quality evidence from evidence-based medicine and translational clinical research, with the aim of enhancing and updating readers' knowledge of neoadjuvant therapy for MIBC and providing insights for future practice and research directions.
Keywords: Clinical trials; Liquid biopsy; Muscle-invasive bladder cancer; Neoadjuvant therapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This project is not applicable to our research. Consent for publication: This project is not applicable to our research. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Two-Step Meta-Analysis.Oncologist. 2016 Jun;21(6):708-15. doi: 10.1634/theoncologist.2015-0440. Epub 2016 Apr 6. Oncologist. 2016. PMID: 27053504 Free PMC article.
-
A Comparative Study of Gemcitabine-Cisplatin vs. Dose-Dense MVAC (Methotrexate, Vinblastine, Doxorubicin, and Cisplatin) as Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Single-Institution Experience.Cureus. 2025 May 30;17(5):e85071. doi: 10.7759/cureus.85071. eCollection 2025 May. Cureus. 2025. PMID: 40585605 Free PMC article.
-
Current State of Bladder Preservation in High Grade Non-Muscle Invasive Bladder Cancer and Localized Muscle Invasive Bladder Cancer.Curr Oncol Rep. 2025 Jun;27(6):761-773. doi: 10.1007/s11912-025-01657-3. Epub 2025 Apr 30. Curr Oncol Rep. 2025. PMID: 40304944 Review.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Avelumab-based neoadjuvant therapy in patients with muscle-invasive bladder cancer (AURA Oncodistinct-004): a phase 2 multicenter clinical trial.J Immunother Cancer. 2025 May 24;13(5):e012045. doi: 10.1136/jitc-2025-012045. J Immunother Cancer. 2025. PMID: 40413024 Free PMC article. Clinical Trial.
References
-
- Lobo N, Afferi L, Moschini M, Mostafid H, Porten S, Psutka SP, Gupta S, Smith AB, Williams SB, Lotan Y. Epidemiology, screening, and prevention of bladder cancer. Eur Urol Oncol. 2022;5:628–39. - PubMed
-
- Alfred Witjes J, Max Bruins H, Carrión A, Cathomas R, Compérat E, Efstathiou JA, Fietkau R, Gakis G, Lorch A, Martini A, Mertens LS, Meijer RP, Milowsky MI, Neuzillet Y, Panebianco V, Redlef J, Rink M, Rouanne M, Thalmann GN, Sæbjørnsen S, Veskimäe E, van der Heijden AG. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2023 guidelines. Eur Urol. 2024;85:17–31. - PubMed
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63. - PubMed
-
- Lobo N, Shariat SF, Guo CC, Fernandez MI, Kassouf W, Choudhury A, Gao J, Williams SB, Galsky MD, Taylor JA, Roupret M, Kamat AM. What is the significance of variant histology in urothelial carcinoma? Eur Urol Focus. 2020;6:653–63. - PubMed
-
- Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, Gontero P, Liedberg F, Masson-Lecomte A, Mostafid AH, Palou J, van Rhijn BWG, Rouprêt M, Shariat SF, Seisen T, Soukup V, Sylvester RJ. European association of urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ). Eur Urol. 2022;81:75–94. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
