Immunomodulatory properties of the gut microbiome: diagnostic and therapeutic potential for rheumatoid arthritis
- PMID: 40591032
- PMCID: PMC12214023
- DOI: 10.1007/s10238-025-01777-x
Immunomodulatory properties of the gut microbiome: diagnostic and therapeutic potential for rheumatoid arthritis
Abstract
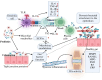
Rheumatoid arthritis (RA) is a chronic autoimmune disorder characterized by persistent joint inflammation, synovial hyperplasia, and progressive joint destruction. Despite advancements in biologic disease-modifying antirheumatic drugs (bDMARDs) and TNF-α blockers, many RA patients still require more effective treatment options. Although genetic and environmental factors play a role in RA development, recent studies have emphasized the influence of the gut microbiota on disease onset and progression. Dysbiosis, or an imbalance in the gut microbial composition, has been linked to immune dysregulation, increased intestinal permeability, and systemic inflammation, all contributing to RA development. Research has revealed changes in the gut microbiome of RA patients, including an increased prevalence of Prevotella copri and a decreased presence of beneficial microbes such as Bifidobacterium, Bacteroides, and Lactobacillus. RA patients exhibit altered metabolite profiles, with reduced levels of short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate, which are linked to immune regulation and intestinal barrier function. Specific metabolites, such as L-arginine, phosphorylcholine, and arachidonic acid, have potential as RA biomarkers, with predictive value for diagnosis. Therapeutic approaches focusing on the microbiome, including probiotics, fecal microbiota transplantation, and traditional medicines, show promise in alleviating RA symptoms and regulating immune function. This review provides an updated overview of the immunomodulatory effects of the gut microbiome and explores its potential applications in the diagnosis and treatment of RA.
Keywords: Diagnostic markers; Immunomodulation; Microbiome; Probiotics; Rheumatoid arthritis; Therapeutics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable.
Figures

References
-
- Yun H, Wang X. Alterations of the intestinal microbiome and metabolome in women with rheumatoid arthritis. Clin Exp Med. 2023;23(8):4695–706. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

